Experimental brain injury induces regionally distinct apoptosis during the acute and delayed post-traumatic period
- PMID: 9671657
- PMCID: PMC6793063
- DOI: 10.1523/JNEUROSCI.18-15-05663.1998
Experimental brain injury induces regionally distinct apoptosis during the acute and delayed post-traumatic period
Abstract
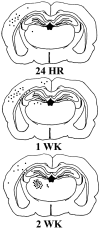
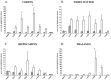
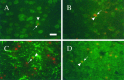
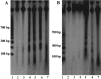
The temporal pattern of apoptosis in the adult rat brain after lateral fluid-percussion (FP) brain injury was characterized using terminal deoxynucleotidyl-transferase-mediated biotin-dUTP nick end labeling (TUNEL) histochemistry and agarose gel electrophoresis. Male Sprague Dawley rats were subjected to brain injury and killed for histological analysis at intervals from 12 hr to 2 months after injury (n = 3/time point). Sham (uninjured) controls were subjected to anesthesia with (n = 3) or without (n = 3) surgery. Apoptotic TUNEL-positive cells were defined using stringent morphological criteria including nuclear shrinkage and fragmentation and condensation of chromatin and cytoplasm. Double-labeled immunocytochemistry was performed to identify TUNEL-positive neurons (anti-neurofilament monoclonal antibody RM044), astrocytes (anti-glial fibrillary acidic protein polyclonal antibody), and oligodendrocytes (anti-cyclic nucleotide phosphohydrolase polyclonal antibody). Compared with that seen with sham controls, in the injured cortex, significant apoptosis occurred at 24 hr (65 +/- 19 cells; p < 0.05) with a second, more pronounced response at 1 week after injury (91 +/- 24 cells; p < 0.05). The number of apoptotic cells in the white matter was increased as early as 12 hr after injury and peaked by 1 week (33 +/- 6 cells; p < 0.05). An increase in apoptotic cells was observed in the hippocampus at 48 hr (13 +/- 8), whereas in the thalamus, the apoptotic response was delayed, peaking at 2 weeks after injury (151 +/- 71 cells; p < 0.05). By 2 months, the number of apoptotic cells in most regions had returned to uninjured levels. At 24 hr after injury, TUNEL-labeled neurons and oligodendrocytes were localized primarily to injured cortex. By 1 week after injury, populations of TUNEL-labeled astrocytes and oligodendrocytes were present in the injured cortex, while double-labeled neurons were present predominantly in injured cortex and thalamus, with a few scattered in the hippocampus. DNA agarose gels confirmed morphological identification of apoptosis. These data suggest that the apoptotic response to trauma is regionally distinct and may be involved in both acute and delayed patterns of cell death.
Figures

Similar articles
-
Mild traumatic brain injury induces apoptotic cell death in the cortex that is preceded by decreases in cellular Bcl-2 immunoreactivity.Neuroscience. 2002;110(4):605-16. doi: 10.1016/s0306-4522(01)00461-4. Neuroscience. 2002. PMID: 11934469
-
Spatial and temporal profile of apoptosis following lateral fluid percussion brain injury.Chin J Traumatol. 2002 Feb;5(1):24-7. Chin J Traumatol. 2002. PMID: 11835752
-
Apoptosis and expression of p53 response proteins and cyclin D1 after cortical impact in rat brain.Brain Res. 1999 Feb 6;818(1):23-33. doi: 10.1016/s0006-8993(98)01204-9. Brain Res. 1999. PMID: 9914434
-
Apoptotic detection methods--from morphology to gene.Prog Histochem Cytochem. 2003;38(3):275-339. doi: 10.1016/s0079-6336(03)80002-5. Prog Histochem Cytochem. 2003. PMID: 12756893 Review.
-
Focus on apoptosis to decipher how alcohol and many other drugs disrupt brain development.Front Pediatr. 2014 Aug 4;2:81. doi: 10.3389/fped.2014.00081. eCollection 2014. Front Pediatr. 2014. PMID: 25136546 Free PMC article. Review. No abstract available.
Cited by
-
Temporal changes of cytochrome P450 (Cyp) and eicosanoid-related gene expression in the rat brain after traumatic brain injury.BMC Genomics. 2013 May 4;14:303. doi: 10.1186/1471-2164-14-303. BMC Genomics. 2013. PMID: 23642095 Free PMC article.
-
Wogonin improves histological and functional outcomes, and reduces activation of TLR4/NF-κB signaling after experimental traumatic brain injury.PLoS One. 2012;7(1):e30294. doi: 10.1371/journal.pone.0030294. Epub 2012 Jan 17. PLoS One. 2012. PMID: 22272328 Free PMC article.
-
Use of Anisotropy, 3D Segmented Atlas, and Computational Analysis to Identify Gray Matter Subcortical Lesions Common to Concussive Injury from Different Sites on the Cortex.PLoS One. 2015 May 8;10(5):e0125748. doi: 10.1371/journal.pone.0125748. eCollection 2015. PLoS One. 2015. PMID: 25955025 Free PMC article.
-
Oxidative stress parameters in different brain structures following lateral fluid percussion injury in the rat.Neurochem Res. 2011 May;36(5):913-21. doi: 10.1007/s11064-011-0424-3. Epub 2011 Feb 19. Neurochem Res. 2011. PMID: 21336819
-
Methylphenidate improves executive functions in patients with traumatic brain injuries: a feasibility trial via the idiographic approach.BMC Neurol. 2020 Mar 19;20(1):103. doi: 10.1186/s12883-020-01663-x. BMC Neurol. 2020. PMID: 32192470 Free PMC article. Clinical Trial.
References
-
- Bramlett HM, Green EJ, Dietrich WD, Busto R, Globus MY-T, Ginsberg MD. Posttraumatic brain hypothermia provides protection from sensorimotor and cognitive behavioral deficits. J Neurotrauma. 1995;12:289–298. - PubMed
-
- Bramlett HM, Dietrich WD, Green EJ, Busto R. Chronic histopathological consequences of fluid-percussion brain injury in rats: effects of post-traumatic hypothermia. Acta Neuropathol (Berl) 1997;93:190–199. - PubMed
-
- Colicos MA, Dash PK. Apoptotic morphology of dentate gyrus granule cells following experimental cortical impact injury in rats: possible role in spatial memory deficits. Brain Res. 1996;739:120–131. - PubMed
-
- Chen J, Graham SH, Chan PH, Lan H, Zhou RL, Simon RP. bcl-2 is expressed in neurons that survive focal ischemia in the rat. NeuroReport. 1995;6:394–398. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
