Mechanisms underlying the early establishment of thalamocortical connections in the rat
- PMID: 9671663
- PMCID: PMC6793053
- DOI: 10.1523/JNEUROSCI.18-15-05723.1998
Mechanisms underlying the early establishment of thalamocortical connections in the rat
Abstract
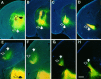
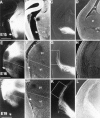
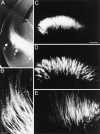
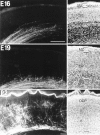
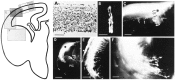
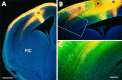
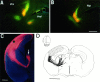
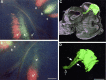
We labeled axonal projections using carbocyanine dyes in the developing rat brain to study cellular interactions that might underlie the establishment of thalamocortical connectivity. By embryonic day 14 (E14), groups of neurons in the ventral diencephalon and the primitive internal capsule have established projections to the dorsal thalamus, and thalamic fibers pass in topographic order among them. Simultaneously, axons from the early-born cells in both subplate and marginal zone (i.e., the original cortical preplate) establish an ordered array that fills the intermediate zone. Thalamic axons and preplate fibers meet in the lateral part of the internal capsule (at E15 for occipital cortex and dorsolateral thalamus). Subsequently, selective labeling of corresponding thalamic and early corticofugal projections reveals thalamic fibers growing in association with early corticofugal axons, right up to the cortical subplate. A small carbocyanine crystal implanted at any point in the cortex shortly after the arrival of thalamic axons (E16 for the occipital cortex) labels a single, tight bundle containing both descending and ascending fibers, rather than two separate tracts, providing further evidence for intimate topographic association of the two axon systems. Crystals placed in a row, parasagittally or coronally along the hemisphere, reveal separate, topographically distributed, discrete fiber bundles throughout the pathway, leading to spatially ordered groups of back-labeled thalamic cells. These results indicate that the topography of thalamic axons is maintained throughout the pathway and that they reach the cortex by associating with the projections of a number of preexisting cells, including the preplate scaffold.
Figures






References
-
- Agmon A, Connors BW. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience. 1991;41:365–379. - PubMed
-
- Bayer SA, Altman J. Neocortical development. Raven; New York: 1991.
-
- Bernardo KL, Woolsey TA. Axonal trajectories between mouse somatosensory thalamus and cortex. J Comp Neurol. 1987;258:542–564. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
