Growth and fate of PSA-NCAM+ precursors of the postnatal brain
- PMID: 9671666
- PMCID: PMC6793045
- DOI: 10.1523/JNEUROSCI.18-15-05777.1998
Growth and fate of PSA-NCAM+ precursors of the postnatal brain
Abstract
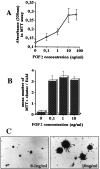
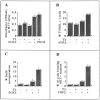
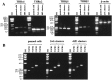
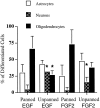
Oligodendrocyte-type 2 astrocyte (O-2A) lineage cells are derived from multipotential stem cells of the developing CNS. Precursors of O-2A progenitors express the polysialylated (PSA) form of the neural cell adhesion molecule (NCAM) and are detected in neonatal rat brain glial cultures. It is unclear how such PSA-NCAM+ "pre-progenitors" are related to neural stem cells and whether they still have the potential to differentiate along several neural lineages. Here we isolated PSA-NCAM+ pre-progenitor cells from glial cultures by immunopanning and found that most of these cells expressed nestin and PDGF-receptor-alpha but not O-2A antigens. PSA-NCAM+ cells synthesized transcripts for fibroblast growth factor (FGF) receptors 1, 2, and 3 and responded to FGF2 by survival and proliferation, growing into large clusters resembling neural spheres. FGF2-induced proliferation of PSA-NCAM+ pre-progenitors was significantly enhanced by thyroid hormone (T3), which on its own did not increase cell survival or mitosis. After adhesion and withdrawal of the mitogen, spheres generated mostly oligodendrocytes and astrocytes but very rarely neurons. PSA-NCAM immunopanned cells grown in epidermal growth factor (EGF) also adopted a mostly glial fate after differentiation. In contrast, PSA-NCAM-negative cells and striatal neonatal stem cells, grown in EGF or FGF2, generated the three CNS cell types. Like neural stem cells, PSA-negative cells generated more oligodendrocytes and fewer neurons when expanded in FGF2 and T3. Thus emergence of PSA-NCAM at the surface of neonatal brain precursors coincides with their restriction to a glial fate. T3 modulates these events by enhancing PSA-NCAM+ pre-progenitor growth in FGF2 and favoring an oligodendrocyte fate.
Figures



References
-
- Ahlgren SC, Wallace H, Bishop J, Neophytou C, Raff MC. Effects of thyroid hormone on embryonic oligodendrocyte precursor cell development in vivo and in vitro. Mol Cell Neurosci. 1997;9:420–432. - PubMed
-
- Almazan G, Honegger P, Matthieu J-M. Triidothyronine stimulation of oligodendroglial differentiation and myelination. Dev Neurosci. 1985;7:45–54. - PubMed
-
- Alvarez-Dolado M, Iglesias T, Rodriguez-Pena A, Bernal J, Munoz A. Expression of neurotrophins and the trk family of neurotrophin receptors in normal and hypothyroid rat brain. Brain Res Mol Brain Res. 1994;27:249–257. - PubMed
-
- Armstrong RC, Harvath L, Dubois-Dalcq ME. Type 1 astrocytes and oligodendrocyte-type 2 astrocyte glial progenitors migrate toward distinct molecules. J Neurosci Res. 1990;27:400–407. - PubMed
-
- Avellana-Adalid V, Nait-Oumesmar B, Lachapelle F, Baron-Van Evercooren A. Expansion of rat oligodendrocyte progenitors into proliferative “oligospheres” that retain differential potential. J Neurosci Res. 1996;45:558–570. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
