Readiness of zebrafish brain neurons to regenerate a spinal axon correlates with differential expression of specific cell recognition molecules
- PMID: 9671667
- PMCID: PMC6793072
- DOI: 10.1523/JNEUROSCI.18-15-05789.1998
Readiness of zebrafish brain neurons to regenerate a spinal axon correlates with differential expression of specific cell recognition molecules
Abstract
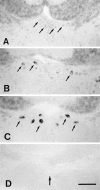
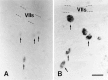
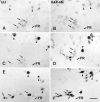
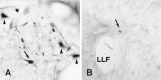
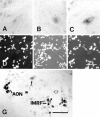
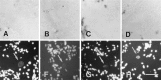
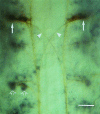
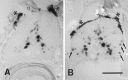
We analyzed changes in the expression of mRNAs for the axonal growth-promoting cell recognition molecules L1.1, L1.2, and neural cell adhesion molecule (NCAM) after a rostral (proximal) or caudal (distal) spinal cord transection in adult zebrafish. One class of cerebrospinal projection nuclei (represented by the nucleus of the medial longitudinal fascicle, the intermediate reticular formation, and the magnocellular octaval nucleus) showed a robust regenerative response after both types of lesions as determined by retrograde tracing and/or in situ hybridization for GAP-43. A second class (represented by the nucleus ruber, the nucleus of the lateral lemniscus, and the tangential nucleus) showed a regenerative response only after proximal lesion. After distal lesion, upregulation of L1.1 and L1.2 mRNAs, but not NCAM mRNA expression, was observed in the first class of nuclei. The second class of nuclei did not show any changes in their mRNA expression after distal lesion. After proximal lesion, both classes of brain nuclei upregulated L1.1 mRNA expression (L1.2 and NCAM were not tested after proximal lesion). In the glial environment distal to the spinal lesion, labeling for L1.2 mRNA but not L1.1 or NCAM mRNAs was increased. These results, combined with findings in the lesioned retinotectal system of zebrafish (Bernharnhardt et al., 1996), indicate that the neuron-intrinsic regulation of cell recognition molecules after axotomy depends on the cell type as well as on the proximity of the lesion to the neuronal soma. Glial reactions differ for different regions of the CNS.
Figures





References
-
- Aigner L, Arber S, Kapfhammer JP, Laux T, Schneider C, Botteri F, Brenner HR, Caroni P. Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell. 1995;83:269–278. - PubMed
-
- Ajemian A, Ness R, David S. Tenascin in the injured rat optic nerve and in non-neuronal cells in vitro: potential role in neural repair. J Comp Neurol. 1994;340:233–242. - PubMed
-
- Bartsch U, Bartsch S, Dorries U, Schachner M. Immunohistological localization of tenascin in the developing and lesioned adult mouse optic nerve. Eur J Neurosci. 1992;4:338–352. - PubMed
-
- Bastmeyer M, Schlosshauer B, Stürmer CAO. The spatio-temporal distribution of N-CAM in the retinotectal pathway of adult goldfish detected by the monoclonal antibody D3. Development. 1990;108:299–311. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
