Immune surveillance in the injured nervous system: T-lymphocytes invade the axotomized mouse facial motor nucleus and aggregate around sites of neuronal degeneration
- PMID: 9671668
- PMCID: PMC6793073
- DOI: 10.1523/JNEUROSCI.18-15-05804.1998
Immune surveillance in the injured nervous system: T-lymphocytes invade the axotomized mouse facial motor nucleus and aggregate around sites of neuronal degeneration
Abstract
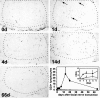
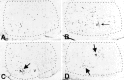
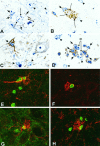
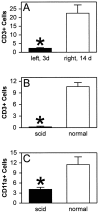
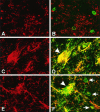
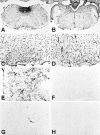
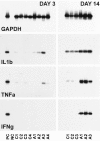
Although the CNS is an established immune-privileged site, it is under surveillance by the immune system, particularly under pathological conditions. In the current study we examined the lymphocyte infiltration, a key component of this neuroimmune surveillance, into the axotomized facial motor nucleus and analyzed the changes in proinflammatory cytokines and the blood-brain barrier. Peripheral nerve transection led to a rapid influx of CD3-, CD11a (alphaL, LFA1alpha)- and CD44-immunoreactive T-cells into the axotomized mouse facial motor nucleus, with a first, low-level plateau 2-4 d after injury, and a second, much stronger increase at 14 d. These T-cells frequently formed aggregates and exhibited typical cleaved lymphocyte nuclei at the EM level. Immunohistochemical colocalization with thrombospondin (TSP), a marker for phagocytotic microglia, revealed aggregation of the T-cells around microglia removing neuronal debris. The massive influx of lymphocytes at day 14 was also accompanied by the synthesis of mRNA encoding IL1beta, TNFalpha, and IFN-gamma. There was no infiltration by the neutrophil granulocytes, and the intravenous injection of horseradish peroxidase also showed an intact blood-brain barrier. However, mice with severe combined immunodeficiency (SCID), which lack differentiated T- and B-cells, still exhibited infiltration with CD11a-positive cells. These CD11a-positive cells also aggregated around phagocytotic microglial nodules. In summary, there is a site-selective infiltration of activated T-cells into the mouse CNS during the retrograde reaction to axotomy. The striking aggregation of these lymphocytes around neuronal debris and phagocytotic microglia suggests an important role for the immune surveillance during neuronal cell death in the injured nervous system.
Figures


Similar articles
-
Endogenous T lymphocytes and microglial reactivity in the axotomized facial motor nucleus of mice: effect of genetic background and the RAG2 gene.J Neuroimmunol. 2006 Mar;172(1-2):1-8. doi: 10.1016/j.jneuroim.2005.10.012. Epub 2006 Jan 10. J Neuroimmunol. 2006. PMID: 16376435
-
Microglial major histocompatibility complex glycoprotein-1 in the axotomized facial motor nucleus: regulation and role of tumor necrosis factor receptors 1 and 2.J Comp Neurol. 2004 Mar 15;470(4):382-99. doi: 10.1002/cne.20017. J Comp Neurol. 2004. PMID: 14961564
-
Age and facial nerve axotomy-induced T cell trafficking: relation to microglial and motor neuron status.Brain Behav Immun. 2011 Jan;25(1):77-82. doi: 10.1016/j.bbi.2010.08.005. Epub 2010 Aug 19. Brain Behav Immun. 2011. PMID: 20727964 Free PMC article.
-
Role of the immune system in the maintenance of mouse facial motoneuron viability after nerve injury.Brain Behav Immun. 2005 Jan;19(1):12-9. doi: 10.1016/j.bbi.2004.05.004. Brain Behav Immun. 2005. PMID: 15581733 Review.
-
Mechanisms and consequences of microglial responses to peripheral axotomy.Front Biosci (Schol Ed). 2011 Jun 1;3(3):857-68. doi: 10.2741/192. Front Biosci (Schol Ed). 2011. PMID: 21622237 Review.
Cited by
-
Increased number of microglia in the brain of severe combined immunodeficient (SCID) mice.Histochem Cell Biol. 2008 Oct;130(4):693-7. doi: 10.1007/s00418-008-0463-2. Epub 2008 Jul 4. Histochem Cell Biol. 2008. PMID: 18600339
-
Microglia as liaisons between the immune and central nervous systems: functional implications for multiple sclerosis.Glia. 2002 Nov;40(2):218-231. doi: 10.1002/glia.10145. Glia. 2002. PMID: 12379909 Free PMC article. Review.
-
Effects of facial nerve axotomy on Th2- and Th1-associated chemokine expression in the facial motor nucleus of wild-type and presymptomatic mSOD1 mice.J Neuroimmunol. 2009 Nov 30;216(1-2):66-75. doi: 10.1016/j.jneuroim.2009.09.009. Epub 2009 Oct 8. J Neuroimmunol. 2009. PMID: 19818514 Free PMC article.
-
Use of laser microdissection in the investigation of facial motoneuron and neuropil molecular phenotypes after peripheral axotomy.Exp Neurol. 2010 Sep;225(1):94-103. doi: 10.1016/j.expneurol.2010.05.019. Epub 2010 Jun 4. Exp Neurol. 2010. PMID: 20570589 Free PMC article.
-
The Role of Microglia in Neuroinflammation of the Spinal Cord after Peripheral Nerve Injury.Cells. 2022 Jun 30;11(13):2083. doi: 10.3390/cells11132083. Cells. 2022. PMID: 35805167 Free PMC article. Review.
References
-
- Adams JC. Heavy metal intensification of DAB-based HRP reaction product. J Histochem Cytochem. 1981;29:775. - PubMed
-
- Aisen PS. Inflammation and Alzheimer disease. Mol Chem Neuropathol. 1996;28:83–88. - PubMed
-
- Akiyama H, McGeer PL. Microglial response to 6-hydroxydopamine-induced substantia nigra lesions. Brain Res. 1989;489:247–253. - PubMed
-
- Banati RB, Rothe G, Valet G, Kreutzberg GW. Detection of lysosomal cysteine proteinases in microglia: flow cytometric measurement and histochemical localization of cathepsin B and L. Glia. 1993;7:183–191. - PubMed
-
- Bancroft GJ, Kelly JP. Macrophage activation and innate resistance to infection in SCID mice. Immunobiology. 1994;191:424–431. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
