Region-specific regulation of glutamic acid decarboxylase (GAD) mRNA expression in central stress circuits
- PMID: 9671680
- PMCID: PMC6793042
- DOI: 10.1523/JNEUROSCI.18-15-05938.1998
Region-specific regulation of glutamic acid decarboxylase (GAD) mRNA expression in central stress circuits
Abstract
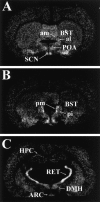
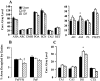
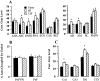
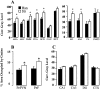
Neurocircuit inhibition of hypothalamic paraventricular nucleus (PVN) neurons controlling hypothalamo-pituitary-adrenocortical (HPA) activity prominently involves GABAergic cell groups of the hypothalamus and basal forebrain. In the present study, stress responsiveness of GABAergic regions implicated in HPA inhibition was assessed by in situ hybridization, using probes recognizing the GABA-synthesizing enzyme glutamic acid decarboxylase (GAD65 and GAD67 isoforms). Acute restraint preferentially increased GAD67 mRNA expression in several stress-relevant brain regions, including the arcuate nucleus, dorsomedial hypothalamic nucleus, medial preoptic area, bed nucleus of the stria terminalis (BST) and hippocampus (CA1 and dentate gyrus). In all cases GAD67 mRNA peaked at 1 hr after stress and returned to unstimulated levels by 2 hr. GAD65 mRNA upregulation was only observed in the BST and dentate gyrus. In contrast, chronic intermittent stress increased GAD65 mRNA in the anterior hypothalamic area, dorsomedial nucleus, medial preoptic area, suprachiasmatic nucleus, anterior BST, perifornical nucleus, and periparaventricular nucleus region. GAD67 mRNA increases were only observed in the medial preoptic area, anterior BST, and hippocampus. Acute and chronic stress did not affect GAD65 or GAD67 mRNA expression in the caudate nucleus, reticular thalamus, or parietal cortex. Overall, the results indicate preferential upregulation of GAD in central circuitry responsible for direct (hypothalamus, BST) or multisynaptic (hippocampus) control of HPA activity. The distinct patterns of GAD65 and GAD67 by acute versus chronic stress suggest stimulus duration-dependent control of GAD biosynthesis. Chronic stress-induced increases in GAD65 mRNA expression predict enhanced availability of GAD65 apoenzyme after prolonged stimulation, whereas acute stress-specific GAD67 upregulation is consistent with de novo synthesis of active enzyme by discrete stressful stimuli.
Figures

Similar articles
-
GABAergic circuits and the stress hyporesponsive period in the rat: ontogeny of glutamic acid decarboxylase (GAD) 67 mRNA expression in limbic-hypothalamic stress pathways.Brain Res. 2007 Mar 23;1138:1-9. doi: 10.1016/j.brainres.2006.04.082. Epub 2007 Feb 5. Brain Res. 2007. PMID: 17276416
-
Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs.J Neurosci. 2007 Feb 21;27(8):2025-34. doi: 10.1523/JNEUROSCI.4301-06.2007. J Neurosci. 2007. PMID: 17314298 Free PMC article.
-
Central stress-integrative circuits: forebrain glutamatergic and GABAergic projections to the dorsomedial hypothalamus, medial preoptic area, and bed nucleus of the stria terminalis.Brain Struct Funct. 2014 Jul;219(4):1287-303. doi: 10.1007/s00429-013-0566-y. Epub 2013 May 10. Brain Struct Funct. 2014. PMID: 23661182 Free PMC article.
-
Role of GABA and glutamate circuitry in hypothalamo-pituitary-adrenocortical stress integration.Ann N Y Acad Sci. 2004 Jun;1018:35-45. doi: 10.1196/annals.1296.004. Ann N Y Acad Sci. 2004. PMID: 15240350 Review.
-
Dopaminergic regulation of glutamic acid decarboxylase mRNA expression and GABA release in the striatum: a review.Prog Neuropsychopharmacol Biol Psychiatry. 1993 Nov;17(6):887-903. doi: 10.1016/0278-5846(93)90018-n. Prog Neuropsychopharmacol Biol Psychiatry. 1993. PMID: 8278600 Review.
Cited by
-
Pituitary Adenylate-Cyclase Activating Polypeptide Regulates Hunger- and Palatability-Induced Binge Eating.Front Neurosci. 2016 Aug 22;10:383. doi: 10.3389/fnins.2016.00383. eCollection 2016. Front Neurosci. 2016. PMID: 27597817 Free PMC article.
-
Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor alpha within the hypothalamus.Neuroscience. 2009 Mar 17;159(2):883-95. doi: 10.1016/j.neuroscience.2008.12.058. Epub 2009 Jan 7. Neuroscience. 2009. PMID: 19166915 Free PMC article.
-
Ghrelin activates hypophysiotropic corticotropin-releasing factor neurons independently of the arcuate nucleus.Psychoneuroendocrinology. 2016 May;67:27-39. doi: 10.1016/j.psyneuen.2016.01.027. Epub 2016 Feb 1. Psychoneuroendocrinology. 2016. PMID: 26874559 Free PMC article.
-
Stress-induced plasticity of GABAergic inhibition.Front Cell Neurosci. 2014 Jun 6;8:157. doi: 10.3389/fncel.2014.00157. eCollection 2014. Front Cell Neurosci. 2014. PMID: 24936173 Free PMC article. Review.
-
Epigenetic and Neuronal Activity Markers Suggest the Recruitment of the Prefrontal Cortex and Hippocampus in the Three-Hit Model of Depression in Male PACAP Heterozygous Mice.Int J Mol Sci. 2022 Oct 3;23(19):11739. doi: 10.3390/ijms231911739. Int J Mol Sci. 2022. PMID: 36233039 Free PMC article.
References
-
- Antoni FA. Hypothalamic control of adrenocorticotropin secretion: advances since the discovery of 41-residue corticotropin-releasing factor. Endocr Rev. 1986;7:351–378. - PubMed
-
- Buijs RM, Kalsbeek A, van der Woude TP, van Heerikhuize JJ, Shinn S. Suprachiasmatic nucleus lesion increases corticosterone secretion. Am J Physiol. 1993a;264:R1186–R1192. - PubMed
-
- Buijs RM, Markman M, Nunes CB, Hou YX, Shinn S. Projections of the suprachiasmatic nucleus to stress-related areas in the rat hypothalamus: a light and electron microscopic study. J Comp Neurol. 1993b;335:42–54. - PubMed
-
- Cascio CS, Shinsako J, Dallman MF. The suprachiasmatic nuclei stimulate evening ACTH secretion in the rat. Brain Res. 1987;423:173–178. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
