Multitasking in the olfactory system: context-dependent responses to odors reveal dual GABA-regulated coding mechanisms in single olfactory projection neurons
- PMID: 9671685
- PMCID: PMC6793051
- DOI: 10.1523/JNEUROSCI.18-15-05999.1998
Multitasking in the olfactory system: context-dependent responses to odors reveal dual GABA-regulated coding mechanisms in single olfactory projection neurons
Abstract
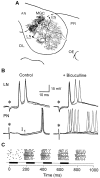
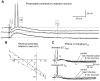
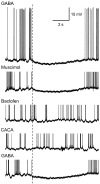
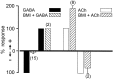
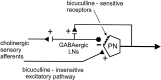
Studies of olfaction have focused mainly on neural processing of information about the chemistry of odors, but olfactory stimuli have other properties that also affect central responses and thus influence behavior. In moths, continuous and intermittent stimulation with the same odor evokes two distinct flight behaviors, but the neural basis of this differential response is unknown. Here we show that certain projection neurons (PNs) in the primary olfactory center in the brain give context-dependent responses to a specific odor blend, and these responses are shaped in several ways by a bicuculline-sensitive GABA receptor. Pharmacological dissection of PN responses reveals that bicuculline blocks GABAA-type receptors/chloride channels in PNs, and that these receptors play a critical role in shaping the responses of these glomerular output neurons. The firing patterns of PNs are not odor-specific but are strongly modulated by the temporal pattern of the odor stimulus. Brief repetitive odor pulses evoke fast inhibitory potentials, followed by discrete bursts of action potentials that are phase-locked to the pulses. In contrast, the response to a single prolonged stimulus with the same odor is a series of slow oscillations underlying irregular firing. Bicuculline disrupts the timing of both types of responses, suggesting that GABAA-like receptors underlie both coding mechanisms. These results suggest that glomerular output neurons could use more than one coding scheme to represent a single olfactory stimulus. Moreover, these context-dependent odor responses encode information about both the chemical composition and the temporal pattern of the odor signal. Together with behavioral evidence, these findings suggest that context-dependent odor responses evoke different perceptions in the brain that provide the animal with important information about the spatiotemporal variations that occur in natural odor plumes.
Figures



References
-
- Adrian ED. The electrical activity of the mammalian olfactory bulb. Electroencephalogr Clin Neurophysiol. 1950;2:377–388. - PubMed
-
- Axel R. The molecular logic of smell. Sci Am. 1995;273:154–159. - PubMed
-
- Baker TC, Haynes KF. Field and laboratory electroantennographic measurements of pheromone plume structure correlated with oriental fruit moth behaviour. Physiol Entomol. 1989;14:1–12.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
