The reverse transcriptase component of the Tetrahymena telomerase ribonucleoprotein complex
- PMID: 9671704
- PMCID: PMC21102
- DOI: 10.1073/pnas.95.15.8485
The reverse transcriptase component of the Tetrahymena telomerase ribonucleoprotein complex
Abstract
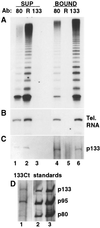
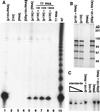
Telomerase is a eukaryotic reverse transcriptase that adds simple sequence repeats to chromosome ends by copying a template sequence within the RNA component of the enzyme. We describe here the identification of a Tetrahymena telomerase protein with reverse transcriptase motifs, p133. This subunit is associated with the previously identified Tetrahymena telomerase RNA and the telomerase proteins p80 and p95 in immunoprecipitation assays. Therefore, all four known Tetrahymena telomerase components are present in a single complex. Expressed in rabbit reticulocyte lysate, recombinant p133 and telomerase RNA alone catalyze a reverse transcriptase activity with some similarities to and some differences from native Tetrahymena telomerase. These experiments suggest a complexity of telomerase structure and function.
Figures


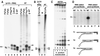
References
-
- Greider C W. Annu Rev Biochem. 1996;66:337–365. - PubMed
-
- Greider C W, Blackburn E H. Nature (London) 1989;337:331–337. - PubMed
-
- Yu G, Bradley J D, Attardi L D, Blackburn E H. Nature (London) 1990;344:126–132. - PubMed
-
- Collins K, Greider C W. Genes Dev. 1993;7:1364–1376. - PubMed
-
- Cohn M, Blackburn E H. Science. 1995;269:396–400. - PubMed
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

