In vivo and in vitro complementation of the N-terminal domain of enzyme I of the Escherichia coli phosphotransferase system by the cloned C-terminal domain
- PMID: 9671705
- PMCID: PMC21103
- DOI: 10.1073/pnas.95.15.8491
In vivo and in vitro complementation of the N-terminal domain of enzyme I of the Escherichia coli phosphotransferase system by the cloned C-terminal domain
Abstract
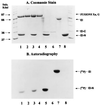
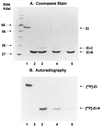
Enzyme I (EI) is the first protein in the phosphoryl transfer sequence from phosphoenolpyruvate (PEP) to sugar in carbohydrate uptake via the bacterial PEP:glycose phosphotransferase system. The EI monomer/dimer transition may regulate the phosphotransferase system because only the EI dimer is autophosphorylated by PEP. We previously showed that the EI monomer comprises two major domains: (i) a compact, protease-resistant N-terminal domain (EI-N), containing the active site His, and (ii) a flexible, protease-sensitive C-terminal domain (EI-C), which is required for EI dimerization. EI-N interacts with the second protein, HPr, and phospho-HPr, but EI-N neither dimerizes nor is phosphorylated by PEP. We report here the molecular cloning and some properties of EI-C. EI-C is rapidly proteolyzed in vivo. Therefore, two different overexpression vectors encoding fusion proteins were constructed. Fusion Xa contains MalE (the maltose-binding protein), the four-amino acid sequence required by protease factor Xa, followed by EI-C. Fusion G contains His-Tyr between MalE and EI-C and is cleaved by the protease genenase. Homogenous EI-C was isolated from fusion G. [32P]PEP phosphorylated EI-N when supplemented with EI-C, fusion Xa, or fusion G. EI-C may act catalytically. Complementation was also demonstrated in vivo. An Escherichia coli ptsI deletion grew on mannitol as the sole source of carbon after it was transformed with two compatible vectors; one vector encoded EI-N and the other encoded fusion Xa or fusion G. The molecular details underlying important properties of EI can now be studied.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

