Gating modifier toxins reveal a conserved structural motif in voltage-gated Ca2+ and K+ channels
- PMID: 9671721
- PMCID: PMC21119
- DOI: 10.1073/pnas.95.15.8585
Gating modifier toxins reveal a conserved structural motif in voltage-gated Ca2+ and K+ channels
Abstract
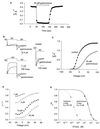
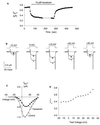
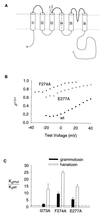
Protein toxins from venomous animals exhibit remarkably specific and selective interactions with a wide variety of ion channels. Hanatoxin and grammotoxin are two related protein toxins found in the venom of the Chilean Rose Tarantula, Phrixotrichus spatulata. Hanatoxin inhibits voltage-gated K+ channels and grammotoxin inhibits voltage-gated Ca2+ channels. Both toxins inhibit their respective channels by interfering with normal operation of the voltage-dependent gating mechanism. The sequence homology of hanatoxin and grammotoxin, as well as their similar mechanism of action, raises the possibility that they interact with the same region of voltage-gated Ca2+ and K+ channels. Here, we show that each toxin can interact with both voltage-gated Ca2+ and K+ channels and modify channel gating. Moreover, mutagenesis of voltage-gated K+ channels suggests that hanatoxin and grammotoxin recognize the same structural motif. We propose that these toxins recognize a voltage-sensing domain or module present in voltage-gated ion channels and that this domain has a highly conserved three-dimensional structure.
Figures
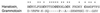

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

