Evidence that phospholipase A2 activity is required for Golgi complex and trans Golgi network membrane tubulation
- PMID: 9671731
- PMCID: PMC21129
- DOI: 10.1073/pnas.95.15.8642
Evidence that phospholipase A2 activity is required for Golgi complex and trans Golgi network membrane tubulation
Abstract
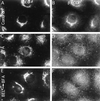
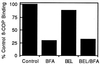
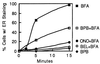
Membrane tubules of uniform diameter (60-80 nm) and various lengths (up to several micrometers) emanate from elements of the Golgi stack and trans Golgi network (TGN). These organelle membrane tubules are thought to be involved in membrane trafficking and maintenance of Golgi/TGN architecture. The number of these tubules, and their frequency of formation, can be greatly enhanced by the fungal metabolite brefeldin A (BFA), an inhibitor of Golgi/TGN-associated coated vesicle formation. We show here that BFA stimulation of Golgi and TGN membrane tubulation, and the resultant retrograde transport of resident Golgi enzymes to the endoplasmic reticulum, was potently inhibited by a number of membrane-permeant antagonists of phospholipase A2 (PLA2; EC 3.1.1.4) activity. In addition, PLA2 inhibitors on their own caused a reversible fragmentation of the Golgi complex into juxtanuclear, stacked cisternal elements. We conclude from these observations that tubulation of Golgi complex and TGN membranes requires a PLA2 activity, and that this activity may participate not only in Golgi tubule-mediated retrograde trafficking to the endoplasmic reticulum, but also in the maintenance of Golgi complex architecture.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

