Targeted oncogenesis of hormone-negative pancreatic islet progenitor cells
- PMID: 9671733
- PMCID: PMC21131
- DOI: 10.1073/pnas.95.15.8654
Targeted oncogenesis of hormone-negative pancreatic islet progenitor cells
Abstract
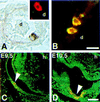
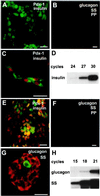
Transgenic mice containing an upstream glucokinase (betaGK) promoter- simian virus 40 T antigen (Tag) fusion gene develop neuroendocrine tumors primarily in the pancreas, gut, and pituitary. Pancreatic tumors from a line with delayed tumorigenesis were of two different types: insulinomas and noninsulinomas. The noninsulinomas are often periductal in location, express none of the four major islet peptide hormones, Glut-2, Pdx1, tyrosine hydroxylase, Pax4, Pax6, or Nkx6.1, but do express glucokinase, Sur1, Isl1, Hnf3beta, Hnf6, Beta2/NeuroD, and Nkx2.2. Cells from two different noninsulinoma tumors, when adapted to culture, began to express either insulin, glucagon, or somatostatin. Given the partial gene expression repertoire of the noninsulinoma tumors, their apparent periductal origin, and the ability of these cells to partially cytodifferentiate in culture, we suggest that these tumors are derived from islet progenitor cells. Thus, betaGK-Tag transgenic mice provide a new model system for studying the events that occur during both islet cell neogenesis and normal embryonic development.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

