Knockout of the abetalipoproteinemia gene in mice: reduced lipoprotein secretion in heterozygotes and embryonic lethality in homozygotes
- PMID: 9671739
- PMCID: PMC21137
- DOI: 10.1073/pnas.95.15.8686
Knockout of the abetalipoproteinemia gene in mice: reduced lipoprotein secretion in heterozygotes and embryonic lethality in homozygotes
Abstract
Abetalipoproteinemia, an inherited human disease characterized by a near-complete absence of the apolipoprotein (apo) B-containing lipoproteins in the plasma, is caused by mutations in the gene for microsomal triglyceride transfer protein (MTP). We used gene targeting to knock out the mouse MTP gene (Mttp). In heterozygous knockout mice (Mttp+/- ), the MTP mRNA, protein, and activity levels were reduced by 50%, in both liver and intestine. Compared with control mice (Mttp+/+), chow-fed Mttp+/- mice had reduced plasma levels of low-density lipoprotein cholesterol and had a 28% reduction in plasma apoB100 levels. On a high-fat diet, the Mttp+/- mice exhibited a marked reduction in total plasma cholesterol levels, compared with those in Mttp+/+ mice. Both the livers of adult Mttp+/- mice and the visceral endoderm of the yolk sacs from Mttp+/- embryos manifested an accumulation of cytosolic fat. All homozygous embryos (Mttp-/-) died during embryonic development. In the visceral endoderm of Mttp-/- yolk sacs, lipoprotein synthesis was virtually absent, and there was a marked accumulation of cytosolic fat droplets. In summary, half-normal MTP levels do not support normal levels of lipoprotein synthesis and secretion, and a complete deficiency of MTP causes lethal developmental abnormalities, perhaps because of an impaired capacity of the yolk sac to export lipids to the developing embryo.
Figures
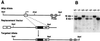
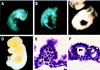
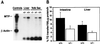
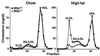
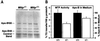
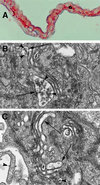
References
-
- Kane J P, Havel R J. In: The Metabolic and Molecular Basis of Inherited Disease. Scriver C R, Beaudet A L, Sly W S, Valle D, editors. New York: McGraw–Hill; 1995. pp. 1853–1885.
-
- Illingworth D R, Connor W E, Miller R G. Arch Neurol. 1980;37:659–662. - PubMed
-
- Kayden H J, Traber M G. J Lipid Res. 1993;34:343–358. - PubMed
-
- Wetterau J R, Aggerbeck L P, Bouma M-E, Eisenberg C, Munck A, Hermier M, Schmitz J, Gay G, Rader D J, Gregg R E. Science. 1992;258:999–1001. - PubMed
-
- Shoulders C C, Brett D J, Bayliss J D, Narcisi T M E, Jarmuz A, Grantham T T, Leoni P R D, Bhattacharya S, Pease R J, Cullen P M, et al. Hum Mol Genet. 1993;2:2109–2116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

