Variable and tissue-specific hormone resistance in heterotrimeric Gs protein alpha-subunit (Gsalpha) knockout mice is due to tissue-specific imprinting of the gsalpha gene
- PMID: 9671744
- PMCID: PMC21142
- DOI: 10.1073/pnas.95.15.8715
Variable and tissue-specific hormone resistance in heterotrimeric Gs protein alpha-subunit (Gsalpha) knockout mice is due to tissue-specific imprinting of the gsalpha gene
Abstract
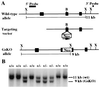
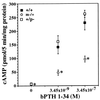
Albright hereditary osteodystrophy (AHO), an autosomal dominant disorder characterized by short stature, obesity, and skeletal defects, is associated with heterozygous inactivating mutations of GNAS1, the gene encoding the heterotrimeric G protein alpha-subunit (Gsalpha) that couples multiple receptors to the stimulation of adenylyl cyclase. It has remained unclear why only some AHO patients present with multihormone resistance and why AHO patients demonstrate resistance to some hormones [e.g., parathyroid hormone (PTH)] but not to others (e.g., vasopressin), even though all activate adenylyl cyclase. We generated mice with a null allele of the mouse homolog Gnas. Homozygous Gs deficiency is embryonically lethal. Heterozygotes with maternal (m-/+) and paternal (+/p-) inheritance of the Gnas null allele have distinct phenotypes, suggesting that Gnas is an imprinted gene. PTH resistance is present in m-/+, but not +/p-, mice. Gsalpha expression in the renal cortex (the site of PTH action) is markedly reduced in m-/+ but not in +/p- mice, demonstrating that the Gnas paternal allele is imprinted in this tissue. Gnas is also imprinted in brown and white adipose tissue. The maximal physiological response to vasopressin (urinary concentrating ability) is normal in both m-/+ and +/p- mice and Gnas is not imprinted in the renal inner medulla (the site of vasopressin action). Tissue-specific imprinting of Gnas is likely the mechanism for variable and tissue-specific hormone resistance in these mice and a similar mechanism might explain the variable phenotype in AHO.
Figures



References
-
- Spiegel A M, Shenker A, Weinstein L S. Endocr Rev. 1992;13:536–565. - PubMed
-
- Gejman P V, Weinstein L S, Martinez M, Spiegel A M, Cao Q, Hsieh W-T, Hoehe M R, Gershon E S. Genomics. 1991;9:782–783. - PubMed
-
- Weinstein L S. In: G Proteins, Receptors, and Disease. Spiegel A M, editor. Totowa, NJ: Humana; 1998. pp. 23–56.
-
- Spiegel A M. N Engl J Med. 1990;322:1461–1462. - PubMed
-
- Bartolomei M S, Tilghman S M. Annu Rev Genet. 1997;31:493–525. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

