Unc-1: a stomatin homologue controls sensitivity to volatile anesthetics in Caenorhabditis elegans
- PMID: 9671752
- PMCID: PMC21150
- DOI: 10.1073/pnas.95.15.8761
Unc-1: a stomatin homologue controls sensitivity to volatile anesthetics in Caenorhabditis elegans
Abstract
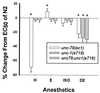
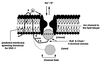
To identify sites of action of volatile anesthetics, we are studying genes in a functional pathway that controls sensitivity to volatile anesthetics in the nematode Caenorhabditis elegans. The unc-1 gene occupies a central position in this pathway. Different alleles of unc-1 have unique effects on sensitivity to the different volatile anesthetics. UNC-1 shows extensive homology to human stomatin, an integral membrane protein thought to regulate an associated ion channel. We postulate that UNC-1 has a direct effect on anesthetic sensitivity in C. elegans and may represent a molecular target for volatile anesthetics.
Figures


References
-
- Franks N P, Lieb W R. Nature (London) 1994;367:607–614. - PubMed
-
- Gruner S M, Shyamsunder E. Ann NY Acad Sci. 1991;625:685–697. - PubMed
-
- Mihic S J, Ye Q, Wick M J, Koltchine W, Krasowski M D, et al. Nature (London) 1997;389:385–389. - PubMed
-
- Koblin D D. In: Anesthesia. 4th Ed. Miller R D, editor. New York: Churchill Livingstone; 1990. pp. 67–99.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

