A second major native von Hippel-Lindau gene product, initiated from an internal translation start site, functions as a tumor suppressor
- PMID: 9671762
- PMCID: PMC21160
- DOI: 10.1073/pnas.95.15.8817
A second major native von Hippel-Lindau gene product, initiated from an internal translation start site, functions as a tumor suppressor
Abstract
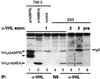
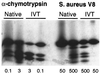
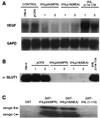
The von Hippel-Lindau (VHL) tumor suppressor gene is inactivated in both sporadic and inherited clear cell renal carcinoma associated with VHL disease. We have identified two distinct native products of the human VHL gene, with apparent molecular masses of 24 and 18 kDa. The 18-kDa VHL protein was more abundant in nearly all cell lines examined. Reintroduction of the 18-kDa VHL gene product into renal carcinoma cells lacking wild-type VHL protein led to down-regulation of vascular endothelial growth factor (VEGF) mRNA and glucose transporter GLUT1 protein and suppressed tumor formation in nude mice. The 18-kDa VHL protein also demonstrated binding to elongins B and C. In an in vitro assay, the second in-frame AUG codon present in VHL mRNA was shown to be necessary and sufficient for production of the 18-kDa VHL protein, consistent with an internal translation mechanism. These data provide evidence for a second major VHL gene product, which contains the functional domains of the VHL gene. Moreover, these results indicate that internal translation initiation is an important mechanism for production of the major VHL protein.
Figures

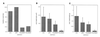

References
-
- Latif F, Tory K, Gnarra J, Yao M, Duh F M, Orcutt M L, Stackhouse T, Kuzmin I, Modi W, Geil L, et al. Science. 1993;260:1317–1320. - PubMed
-
- Linehan W M, Lerman M I, Zbar B. J Am Med Assoc. 1995;273:564–570. - PubMed
-
- Gnarra J R, Tory K, Weng Y, Schmidt L, Wei M H, Li H, Latif F, Liu S, Chen F, Duh F M, et al. Nat Genet. 1994;7:85–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

