Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America
- PMID: 9671799
- PMCID: PMC21197
- DOI: 10.1073/pnas.95.15.9031
Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America
Abstract
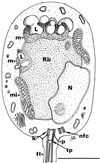
Epidermal changes caused by a chytridiomycete fungus (Chytridiomycota; Chytridiales) were found in sick and dead adult anurans collected from montane rain forests in Queensland (Australia) and Panama during mass mortality events associated with significant population declines. We also have found this new disease associated with morbidity and mortality in wild and captive anurans from additional locations in Australia and Central America. This is the first report of parasitism of a vertebrate by a member of the phylum Chytridiomycota. Experimental data support the conclusion that cutaneous chytridiomycosis is a fatal disease of anurans, and we hypothesize that it is the proximate cause of these recent amphibian declines.
Figures



References
-
- Blaustein A R, Wake D B. Trends Ecol Evol. 1990;5:203–204.
-
- Wake D B. Science. 1991;253:860. - PubMed
-
- Drost C A, Fellers G M. Conserv Biol. 1996;10:414–425.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

