Role of a cytotoxic enterotoxin in Aeromonas-mediated infections: development of transposon and isogenic mutants
- PMID: 9673226
- PMCID: PMC108379
- DOI: 10.1128/IAI.66.8.3501-3509.1998
Role of a cytotoxic enterotoxin in Aeromonas-mediated infections: development of transposon and isogenic mutants
Abstract
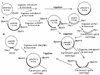
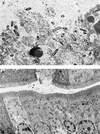
Transposon and marker exchange mutagenesis were used to evaluate the role of Aeromonas cytotoxic enterotoxin (Act) in the pathogenesis of diarrheal diseases and deep wound infections. The transposon mutants were generated by random insertion of Tn5-751 in the chromosomal DNA of a diarrheal isolate SSU of Aeromonas hydrophila. Some of the transposon mutants had dramatically reduced hemolytic and cytotoxic activities, and such mutants exhibited reduced virulence in mice compared to wild-type Aeromonas when injected intraperitoneally (i.p.). Southern blot data indicated that transposition in these mutants did not occur within the cytotoxic enterotoxin gene (act). The transcription of the act gene was affected drastically in the transposon mutants, as revealed by Northern blot analysis. The altered virulence of these transposon mutants was confirmed by developing isogenic mutants of the wild-type Aeromonas by using a suicide vector. In these mutants, the truncated act gene was integrated in place of a functionally active act gene. The culture filtrates from isogenic mutants were devoid of hemolytic, cytotoxic, and enterotoxic activities associated with Act. These filtrates caused no damage to mouse small intestinal epithelium, as determined by electron microscopy, whereas culture filtrates from wild-type Aeromonas caused complete destruction of the microvilli. The 50% lethal dose of these mutants in mice was 1.0 x 10(8) when injected i. p., compared to 3.0 x 10(5) for the wild-type Aeromonas. Reintegration of the native act gene in place of the truncated toxin gene in isogenic mutants resulted in complete restoration of Act's biological activity and virulence in mice. The animals injected with a sublethal dose of wild-type Aeromonas or the revertant, but not the isogenic mutant, had circulating toxin-specific neutralizing antibodies. Taken together, these studies clearly established a role for Act in the pathogenesis of Aeromonas-mediated infections.
Figures



Comment in
-
The cytotoxic enterotoxin of Aeromonas hydrophila is aerolysin.Infect Immun. 1999 Jan;67(1):466-7. doi: 10.1128/IAI.67.1.466-467.1999. Infect Immun. 1999. PMID: 9925450 Free PMC article. No abstract available.
References
-
- Altwegg M, Geiss H K. Aeromonas as a human pathogen. Crit Rev Microbiol. 1989;16:253–286. - PubMed
-
- Altwegg M, Lucchini G M, Luthy-Hottenstein J, Rohrbach M. Aeromonas-associated gastroenteritis after consumption of contaminated shrimp. Eur J Clin Microbiol Infect Dis. 1991;10:44–45. - PubMed
-
- Austin B, Altwegg M, Gosling P J, Joseph S, editors. The genus Aeromonas. New York, N.Y: John Wiley and Sons, Inc.; 1996.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

