Receptor-mediated recognition and uptake of iron from human transferrin by Staphylococcus aureus and Staphylococcus epidermidis
- PMID: 9673237
- PMCID: PMC108390
- DOI: 10.1128/IAI.66.8.3591-3596.1998
Receptor-mediated recognition and uptake of iron from human transferrin by Staphylococcus aureus and Staphylococcus epidermidis
Abstract
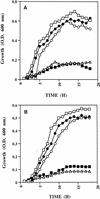
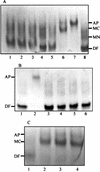
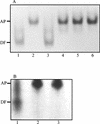
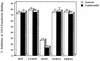
Staphylococcus aureus and Staphylococcus epidermidis both recognize and bind the human iron-transporting glycoprotein, transferrin, via a 42-kDa cell surface protein receptor. In an iron-deficient medium, staphylococcal growth can be promoted by the addition of human diferric transferrin but not human apotransferrin. To determine whether the staphylococcal transferrin receptor is involved in the removal of iron from transferrin, we employed 6 M urea-polyacrylamide gel electrophoresis, which separates human transferrin into four forms (diferric, monoferric N-lobe, and monoferric C-lobe transferrin and apotransferrin). S. aureus and S. epidermidis but not Staphylococcus saprophyticus (which lacks the transferrin receptor) converted diferric human transferrin into its apotransferrin form within 30 min. During conversion, iron was removed sequentially from the N lobe and then from the C lobe. Metabolic poisons such as sodium azide and nigericin inhibited the release of iron from human transferrin, indicating that it is an energy-requiring process. To demonstrate that this process is receptor rather than siderophore mediated, we incubated (i) washed staphylococcal cells and (ii) the staphylococcal siderophore, staphyloferrin A, with porcine transferrin, a transferrin species which does not bind to the staphylococcal receptor. While staphyloferrin A removed iron from both human and porcine transferrins, neither S. aureus nor S. epidermidis cells could promote the release of iron from porcine transferrin. In competition binding assays, both native and recombinant N-lobe fragments of human transferrin as well as a naturally occurring human transferrin variant with a mutation in the C-lobe blocked binding of 125I-labelled transferrin. Furthermore, the staphylococci removed iron efficiently from the iron-loaded N-lobe fragment of human transferrin. These data demonstrate that the staphylococci efficiently remove iron from transferrin via a receptor-mediated process and provide evidence to suggest that there is a primary receptor recognition site on the N-lobe of human transferrin.
Figures


References
-
- Aisen P. Physical biochemistry of the transferrins: update, 1984–1988. Phys Bioinorg Chem Ser. 1989;5:353–371.
-
- Alcantara J, Schryvers A B. Transferrin binding protein 2 interacts with both the N-lobe and C-lobe of ovotransferrin. Microb Pathog. 1996;209:73–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

