Regulation of hly expression in Listeria monocytogenes by carbon sources and pH occurs through separate mechanisms mediated by PrfA
- PMID: 9673243
- PMCID: PMC108396
- DOI: 10.1128/IAI.66.8.3635-3642.1998
Regulation of hly expression in Listeria monocytogenes by carbon sources and pH occurs through separate mechanisms mediated by PrfA
Abstract
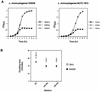
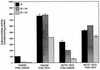
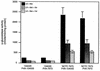
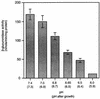
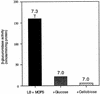
Expression of the PrfA-controlled virulence gene hly (encoding the pore-forming cytolysin listeriolysin) is under negative regulation by readily metabolized carbon sources in Listeria monocytogenes. However, the hyperhemolytic strain NCTC 7973 exhibits deregulated hly expression in the presence of repressing sugars, raising the possibility that a defect in carbon source regulation is responsible for its anomalous behavior. We show here that the activity of a second glucose-repressed enzyme, alpha-glucosidase, is 10-fold higher in NCTC 7973 than in 10403S. Using hly-gus fusions, we show that the prfA allele from NCTC 7973 causes deregulated hly-gus expression in the presence of sugars in either the wild-type or the NCTC 7973 background, while the 10403S prfA allele restores carbon source regulation. However, the prfA genotype does not affect the regulation of alpha-glucosidase activity by repressing sugars. Of the two mutational differences in PrfA, only a Gly145Ser change is important for regulation of hly-gus. Therefore, NCTC 7973 and 10403S have genetic differences in at least two loci: one in prfA that affects carbon source regulation of virulence genes and another in an unidentified gene(s) that up-regulates alpha-glucosidase activity. We also show that the decrease in pH associated with utilization of sugars negatively regulates hly-gus expression, although sugars can affect hly-gus expression by another mechanism that is independent of pH.
Figures


References
-
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1994.
-
- Barak I, Behari J, Olmedo G, Guzman P, Brown D P, Castro E, Walker D, Westpheling J, Youngman P. Structure and function of the Bacillus SpoIIE protein and its localization to sites of sporulation septum assembly. Mol Microbiol. 1996;19:1047–1060. - PubMed
-
- Bielecki J, Youngman P, Connelly P, Portnoy D A. Bacillus subtilis expressing a haemolysin gene from Listeria monocytogenes can grow in mammalian cells. Nature. 1990;345:175–176. - PubMed
-
- Bockmann R, Dickneite C, Middendorf B, Goebel W, Sokolovic Z. Specific binding of the Listeria monocytogenes transcriptional regulator PrfA to target sequences requires additional factor(s) and is influenced by iron. Mol Microbiol. 1996;22:643–653. - PubMed
-
- Bohne J, Kestler H, Uebele C, Sokolovic Z, Goebel W. Differential regulation of the virulence genes of Listeria monocytogenes by the transcriptional activator PrfA. Mol Microbiol. 1996;20:1189–1198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

