Listeria monocytogenes stimulates mucus exocytosis in cultured human polarized mucosecreting intestinal cells through action of listeriolysin O
- PMID: 9673248
- PMCID: PMC108401
- DOI: 10.1128/IAI.66.8.3673-3681.1998
Listeria monocytogenes stimulates mucus exocytosis in cultured human polarized mucosecreting intestinal cells through action of listeriolysin O
Abstract
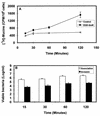
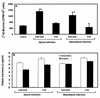
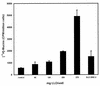
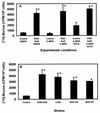
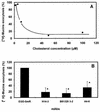
When the intracellular pathogen Listeria monocytogenes infects cultured human mucosecreting polarized HT29-MTX cells apically, it induces the stimulation of mucus exocytosis without cell entry. Using a set of isogenic mutants and purified listeriolysin O (LLO), we identified the L. monocytogenes thiol-activated exotoxin LLO as the agonist of mucus secretion. We demonstrated that the LLO-induced mucus exocytosis did not result from the LLO membrane-damaging activity. We found that LLO-induced mucus exocytosis is an event requiring the binding of LLO to a brush border-associated receptor and membrane oligomerization of the exotoxin. By a pharmacological approach, we demonstrated that no regulatory system or intracellular transducing signal known to be involved in control of mucin exocytosis was activated by LLO. Based on the present data, the stimulatory action of LLO on mucin exocytosis could be accounted for either by an unknown signaling system which remains to be determined or by direct action of LLO with the membrane vesicle components involved in the intracellular vesicular transport of mucins.
Figures

References
-
- Alouf J E, Geoffroy C. Structure activity relationships in sulphydryl-activated toxins. In: Allouf J E, Fehrenbach F J, Freer J H, Jeljaszewicz J, editors. Bacterial protein toxins. London, United Kingdom: Academic Press, Ltd.; 1984. pp. 165–171.
-
- Augeron C, Voisin T, Maoret J J, Berthon B, Laburthe M, Laboisse C. Neurotensin and neuromedin N stimulate mucin ouput in the human goblet cell clone Cl.16E: a neurotensin receptor-mediated event. Am J Physiol. 1992;262:G470–G476. - PubMed
-
- Beubler E, Kollar G, Saria A, Bukhave K, Rask-Madsen J. Involvement of 5-hydroxytryptamine, prostaglandin-E2, and cyclic adenosine monophosphate in cholera toxin-induced fluid secretion in the small intestine of the rat in vivo. Gastroenterology. 1989;96:368–376. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

