Phospholipid composition of purified Chlamydia trachomatis mimics that of the eucaryotic host cell
- PMID: 9673255
- PMCID: PMC108408
- DOI: 10.1128/IAI.66.8.3727-3735.1998
Phospholipid composition of purified Chlamydia trachomatis mimics that of the eucaryotic host cell
Abstract
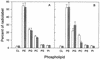
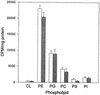
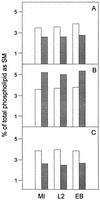
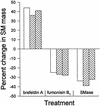
Chlamydia trachomatis is an obligate intracellular eubacterial parasite capable of infecting a wide range of eucaryotic host cells. Purified chlamydiae contain several lipids typically found in eucaryotes, and it has been established that eucaryotic lipids are transported from the host cell to the parasite. In this report, we examine the phospholipid composition of C. trachomatis purified from host cells grown under a variety of conditions in which the cellular phospholipid composition was altered. A mutant CHO cell line, with a thermolabile CDP-choline synthetase, was used to show that decreased host cell phosphatidylcholine levels had no significant effect on C. trachomatis growth. However, less phosphatidylcholine was transported to the parasite and purified elementary bodies contained decreased levels of phosphatidylcholine. Brefeldin A, fumonisin B1, and exogenous sphingomyelinase were used to alter levels of host cell sphingomyelin. None of the agents had a significant effect on C. trachomatis replication. Treatment with fumonisin B1 and exogenous sphingomyelinase resulted in decreased levels of host cell sphingomyelin. This had no effect on glycerophospholipid trafficking to chlamydiae; however, sphingomyelin trafficking was reduced and elementary bodies purified from treated cells had reduced sphingomyelin content. Exposure to brefeldin A, which had no adverse effect on chlamydia growth, resulted in an increase in cellular levels of sphingomyelin and a concomitant increase in the amount of sphingomyelin in purified chlamydiae. Under the experimental conditions used, brefeldin A treatment had only a small effect on sphingomyelin trafficking to the host cell surface or to C. trachomatis. Thus, the final phospholipid composition of purified C. trachomatis mimics that of the host cell in which it is grown.
Figures





References
-
- Andrieu N, Salvayre R, Levade T. Comparative study of the metabolic pools of sphingomyelin and phosphatidylcholine sensitive to tumor necrosis factor. Eur J Biochem. 1996;236:738–745. - PubMed
-
- Badiani K, Byers D M, Cook H W, Ridgway N D. Effect of fumonisin B1 on phosphatidylethanolamine biosynthesis in Chinese hamster ovary cells. Biochim Biophys Acta. 1996;1304:190–196. - PubMed
-
- Beatty P R, Stephens R S. CD+ T-lymphocyte mediated lysis of Chlamydia-infected L cells using an endogenous antigen pathway. J Immunol. 1994;153:4588–4595. - PubMed
-
- Belunis C J, Mdluli K E, Raetz C R H, Nano F E. A novel 3-deoxy-d-manno-octulosonic acid transferase from Chlamydia trachomatis required for expression of the genus specific epitope. J Biol Chem. 1992;267:18702–18707. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

