Molecular cloning of a 32-kilodalton lipoprotein component of a novel iron-regulated Staphylococcus epidermidis ABC transporter
- PMID: 9673260
- PMCID: PMC108413
- DOI: 10.1128/IAI.66.8.3767-3774.1998
Molecular cloning of a 32-kilodalton lipoprotein component of a novel iron-regulated Staphylococcus epidermidis ABC transporter
Abstract
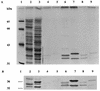
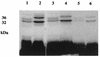
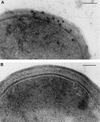
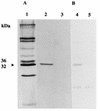
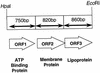
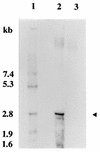
Our previous studies identified two iron-regulated cytoplasmic membrane proteins of 32 and 36 kDa expressed by both Staphylococcus epidermidis and Staphylococcus aureus. In this study we show by Triton X-114 phase partitioning and tritiated palmitic acid labelling that these proteins are lipoproteins which are anchored into the cytoplasmic membrane by their lipid-modified N termini. In common with those of some other gram-positive bacteria, these highly immunogenic lipoproteins were released from the bacterial cell into the culture supernatants, with release being promoted by growth of the bacteria under iron-restricted conditions. Immunoelectron microscopy with a monospecific rabbit antiserum to the 32-kDa S. epidermidis lipoprotein showed that the majority of the antigen was distributed throughout the staphylococcal cell wall. Only minor quantities were detected in the cytoplasmic membrane, and exposure of the lipoprotein on the bacterial surface was minimal. A monoclonal antibody raised to the 32-kDa lipoprotein of S. aureus was used in immunoblotting studies to investigate the conservation of this antigen among a variety of staphylococci. The monoclonal antibody reacted with polypeptides of 32 kDa in S. epidermidis and S. aureus and of 40 kDa in Staphylococcus hominis. No reactivity was detected with Staphylococcus lugdunensis, Staphylococcus cohni, or Staphylococcus haemolyticus. The gene encoding the 32-kDa lipoprotein from S. epidermidis has been isolated from a Lambda Zap II genomic DNA library and found to be a component of an iron-regulated operon encoding a novel ABC-type transporter. The operon contains three genes, designated sitA, -B, and -C, encoding an ATPase, a cytoplasmic membrane protein, and the 32-kDa lipoprotein, respectively. SitC shows significant homology both with a number of bacterial adhesins, including FimA of Streptococcus parasanguis and ScaA of Streptococcus gordonii, and with lipoproteins of a recently described family of ABC transporters with proven or putative metal ion transport functions. Although the solute specificity of this novel transporter has not yet been determined, we speculate that it may be involved in either siderophore- or transferrin-mediated iron uptake in S. epidermidis.
Figures
References
-
- Arbuthnott J P, Arbuthnott E, Arbuthnott A D J, Pike W J, Cockayne A. Investigation of microbial growth in vivo: evaluation of a novel in vivo chamber implant system. FEMS Microbiol Lett. 1992;100:75–80. - PubMed
-
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates; 1994.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

