Hemolysin-positive enteroaggregative and cell-detaching Escherichia coli strains cause oncosis of human monocyte-derived macrophages and apoptosis of murine J774 cells
- PMID: 9673280
- PMCID: PMC108451
- DOI: 10.1128/IAI.66.8.3918-3924.1998
Hemolysin-positive enteroaggregative and cell-detaching Escherichia coli strains cause oncosis of human monocyte-derived macrophages and apoptosis of murine J774 cells
Abstract
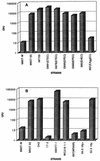
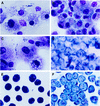
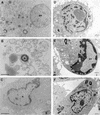
Infection of human monocyte-derived macrophages (HMDM) and J774 cells (murine macrophage cell line) with several enteroaggregative and cytodetaching Escherichia coli (EAggEC and CDEC, respectively) strains demonstrated that some strains could induce macrophage cell death accompanied by release of lactate dehydrogenase activity and interleukin 1beta (IL-1beta) into culture supernatants. The mode of cell death differed in the two types of macrophages. Damage to macrophage plasma membrane integrity without changes in nuclear morphology resulted in cytolysis of HMDM. This mechanism of cell death has been previously described for virulent Shigella infection of HMDM and is termed oncosis. In contrast, infection of J774 cells by EAggEC and CDEC strains resulted in apoptosis. The presence of alpha-hemolysin (Hly) in EAggEC and CDEC strains appears to be critical for both oncosis in HMDM and apoptosis in J774 cells. Bacteria lacking Hly, including Hly- EAggEC strains as well as enterotoxigenic, enteropathogenic, and enterohemorrhagic E. coli strains, behaved like avirulent Shigella flexneri in that the macrophage monolayers were intact, with no release of lactate dehydrogenase activity or IL-1beta into the culture supernatants.
Figures

References
-
- Abe C M, Marques L R M, Gomes T A T. Abstracts of the 97th Meeting of the American Society for Microbiology. Washington, D.C: American Society for Microbiology; 1997. Alpha-hemolysin production and cell-detaching activity in fecal Escherichia coli strains harbouring DNA sequence(s) associated with putative or established enteropathogenic categories of E. coli, abstr. B-154.
-
- Baudry B, Savarino S J, Vial P, Kaper J B, Levine M M. A sensitive and specific DNA probe to identify enteroaggregative Escherichia coli, a recently discovered diarrheal pathogen. J Infect Dis. 1990;161:1249–1251. - PubMed
-
- Beutin L. The different hemolysins of Escherichia coli. Med Microbiol Immunol. 1991;180:167–182. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

