The tumor suppressor Smad4/DPC4 and transcriptional adaptor CBP/p300 are coactivators for smad3 in TGF-beta-induced transcriptional activation
- PMID: 9679060
- PMCID: PMC317015
- DOI: 10.1101/gad.12.14.2153
The tumor suppressor Smad4/DPC4 and transcriptional adaptor CBP/p300 are coactivators for smad3 in TGF-beta-induced transcriptional activation
Abstract
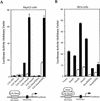
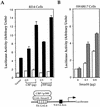
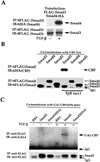
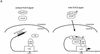
Smads regulate transcription of defined genes in response to TGF-beta receptor activation, although the mechanisms of Smad-mediated transcription are not well understood. We demonstrate that the TGF-beta-inducible Smad3 uses the tumor suppressor Smad4/DPC4 and CBP/p300 as transcriptional coactivators, which associate with Smad3 in response to TGF-beta. The association of CBP with Smad3 was localized to the carboxyl terminus of Smad3, which is required for transcriptional activation, and a defined segment in CBP. Furthermore, CBP/p300 stimulated both TGF-beta- and Smad-induced transcription in a Smad4/DPC4-dependent fashion. Smad3 transactivation and TGF-beta-induced transcription were inhibited by expressing E1A, which interferes with CBP functions. The coactivator functions and physical interactions of Smad4 and CBP/p300 with Smad3 allow a model for the induction of gene expression in response to TGF-beta.
Figures










References
-
- Abdollah S, Macías-Silva M, Tsukazaki T, Hayashi H, Attisano L, Wrana JL. TβRI phosphorylation of Smad2 on Ser465 and Ser467 is required for Smad2-Smad4 complex formation and signaling. J Biol Chem. 1997;272:27678–27685. - PubMed
-
- Avantaggiati ML, Ogryzko V, Gardner K, Giordano A, Levine A, Kelly K. Recruitment of p300/CBP in p53-dependent signal pathways. Cell. 1997;89:1175–1184. - PubMed
-
- Candia A, Watabe T, Hawley S, Onichtchouk D, Zhang Y, Derynck R, Niehrs C, Cho KWY. Cellular interpretation of multiple TGF-β signals: Intracellular antagonism between activin/BVg1 and BMP-2/4 signaling mediated by Smads. Development. 1997;124:4467–4480. - PubMed
-
- Chen X, Weisberg E, Fridmacher V, Watanabe M, Naco G, Whitman M. Smad4 and FAST-1 in the assembly of activin-responsive factor. Nature. 1997;389:85–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
