Impaired megakaryopoiesis and behavioral defects in mafG-null mutant mice
- PMID: 9679061
- PMCID: PMC317009
- DOI: 10.1101/gad.12.14.2164
Impaired megakaryopoiesis and behavioral defects in mafG-null mutant mice
Abstract
The small Maf proteins (MafG, MafK, and MafF), which serve as heterodimeric partner molecules of CNC family proteins for binding in vitro to MARE sites, have been implicated in the regulation of both transcription and chromatin structure, but there is no current evidence that the proteins fulfill these functions in vivo. To elucidate possible contributions of the small Maf proteins to gene regulation, we have ablated the mafG and mafK genes in mice by replacing their entire coding sequences with the Escherichia coli lacZ gene. mafG homozygous mutant animals exhibit impaired platelet formation accompanied by megakaryocyte proliferation, as well as behavioral abnormalities, whereas mafK-null mutant mice are phenotypically normal. Characterization of the mafG and mafK embryonic expression patterns show that their developmental programs are distinct and intersecting, but not entirely overlapping. These results provide direct evidence that the small Maf transcription factors are vital participants in embryonic development and cellular differentiation.
Figures
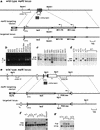

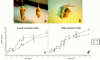
 ) and homozygous (♦) mutant littermates were weighed and averaged from the first week after birth up to 11 weeks of age.
) and homozygous (♦) mutant littermates were weighed and averaged from the first week after birth up to 11 weeks of age.

Similar articles
-
Differential induction of mafF, mafG and mafK expression by electrophile-response-element activators.Biochem J. 2002 Jan 15;361(Pt 2):371-7. doi: 10.1042/0264-6021:3610371. Biochem J. 2002. PMID: 11772409 Free PMC article.
-
Genetic evidence that small maf proteins are essential for the activation of antioxidant response element-dependent genes.Mol Cell Biol. 2005 Sep;25(18):8044-51. doi: 10.1128/MCB.25.18.8044-8051.2005. Mol Cell Biol. 2005. PMID: 16135796 Free PMC article.
-
Perinatal synthetic lethality and hematopoietic defects in compound mafG::mafK mutant mice.EMBO J. 2000 Mar 15;19(6):1335-45. doi: 10.1093/emboj/19.6.1335. EMBO J. 2000. PMID: 10716933 Free PMC article.
-
Small Maf proteins (MafF, MafG, MafK): History, structure and function.Gene. 2016 Jul 25;586(2):197-205. doi: 10.1016/j.gene.2016.03.058. Epub 2016 Apr 5. Gene. 2016. PMID: 27058431 Free PMC article. Review.
-
The world according to Maf.Nucleic Acids Res. 1997 Aug 1;25(15):2953-59. doi: 10.1093/nar/25.15.2953. Nucleic Acids Res. 1997. PMID: 9224592 Free PMC article. Review.
Cited by
-
Differential induction of mafF, mafG and mafK expression by electrophile-response-element activators.Biochem J. 2002 Jan 15;361(Pt 2):371-7. doi: 10.1042/0264-6021:3610371. Biochem J. 2002. PMID: 11772409 Free PMC article.
-
Genetic evidence that small maf proteins are essential for the activation of antioxidant response element-dependent genes.Mol Cell Biol. 2005 Sep;25(18):8044-51. doi: 10.1128/MCB.25.18.8044-8051.2005. Mol Cell Biol. 2005. PMID: 16135796 Free PMC article.
-
Embryonic lethality and fetal liver apoptosis in mice lacking all three small Maf proteins.Mol Cell Biol. 2012 Feb;32(4):808-16. doi: 10.1128/MCB.06543-11. Epub 2011 Dec 12. Mol Cell Biol. 2012. PMID: 22158967 Free PMC article.
-
One enhancer mediates mafK transcriptional activation in both hematopoietic and cardiac muscle cells.EMBO J. 2000 Jun 15;19(12):2980-91. doi: 10.1093/emboj/19.12.2980. EMBO J. 2000. PMID: 10856242 Free PMC article.
-
Zinc finger protein, Hzf, is required for megakaryocyte development and hemostasis.J Exp Med. 2002 Apr 1;195(7):941-52. doi: 10.1084/jem.20011522. J Exp Med. 2002. PMID: 11927637 Free PMC article.
References
-
- Andrews NC, Erjument-Bromage H, Davidson MB, Tempst P, Orkin SH. Erythroid transcription factor NF-E2 is a haematopoietic-specific basic-leucine zipper protein. Nature. 1993a;362:722–728. - PubMed
-
- Beckstead JH, Stenberg PE, McEver RP, Shuman MA, Bainton DF. Immunohistochemical localization of membrane and alpha-granule proteins in human megakaryocytes: Application to plastic-embedded bone marrow biopsy specimens. Blood. 1986;67:285–293. - PubMed
-
- Blank V, Andrews NC. The Maf transcription factors: Regulators of differentiation. Trends Biol Sci. 1997;22:437–441. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
