Mistargeting of the lectin ERGIC-53 to the endoplasmic reticulum of HeLa cells impairs the secretion of a lysosomal enzyme
- PMID: 9679138
- PMCID: PMC2133042
- DOI: 10.1083/jcb.142.2.377
Mistargeting of the lectin ERGIC-53 to the endoplasmic reticulum of HeLa cells impairs the secretion of a lysosomal enzyme
Abstract
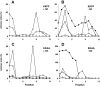
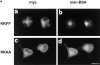
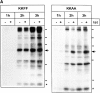
ERGIC-53, a homo-oligomeric recycling protein associated with the ER-Golgi intermediate compartment (ERGIC), has properties of a mannose-selective lectin in vitro, suggesting that it may function as a transport receptor for glycoproteins in the early secretory pathway. To investigate if ERGIC-53 is involved in glycoprotein secretion, a mutant form of this protein was generated that is incapable of leaving the ER. If expressed in HeLa cells in a tetracycline-inducible manner, this mutant accumulated in the ER and retained the endogenous ERGIC-53 in this compartment, thus preventing its recycling. Mistargeting of ERGIC-53 to the ER did not alter the gross morphology of the early secretory pathway, including the distribution of beta'-COP. However, it impaired the secretion of one major glycoprotein, identified as the precursor of the lysosomal enzyme cathepsin C, while overexpression of wild-type ERGIC-53 had no effect on glycoprotein secretion. Transport of two other lysosomal enzymes and three post-Golgi membrane glycoproteins was unaffected by inactivating the recycling of ERGIC-53. The results suggest that the recycling of ERGIC-53 is required for efficient intracellular transport of a small subset of glycoproteins, but it does not appear to be essential for the majority of glycoproteins.
Figures














References
-
- Arar C, Carpentier V, Le Cear J-P, Monsigny M, Legrand A, Roche AC. ERGIC-53, a membrane protein of the endoplasmic reticulum-Golgi intermediate compartment, is identical to MR60, an intracellular mannose-specific lectin of myelomonocytic cells. J Biol Chem. 1995;270:3551–3553. - PubMed
-
- Balch WE, McCaffery JM, Plutner H, Farquhar MG. Vesicular stomatitis virus glycoprotein is sorted and concentrated during export from the endoplasmic reticulum. Cell. 1994;76:841–852. - PubMed

