Iqg1p, a yeast homologue of the mammalian IQGAPs, mediates cdc42p effects on the actin cytoskeleton
- PMID: 9679143
- PMCID: PMC2133066
- DOI: 10.1083/jcb.142.2.443
Iqg1p, a yeast homologue of the mammalian IQGAPs, mediates cdc42p effects on the actin cytoskeleton
Abstract
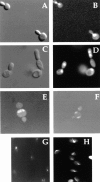
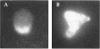
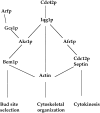
The Rho-type GTPase Cdc42p has been implicated in diverse cellular functions including cell shape, cell motility, and cytokinesis, all of which involve the reorganization of the actin cytoskeleton. Targets of Cdc42p that interface the actin cytoskeleton are likely candidates for mediating cellular activities. In this report, we identify and characterize a yeast homologue for the mammalian IQGAP, a cytoskeletal target for Cdc42p. The yeast IQGAP homologue, designated Iqg1p, displays a two-hybrid interaction with activated Cdc42p and coimmunoprecipitates with actin filaments. Deletion of IQG1 results in a temperature-sensitive lethality and causes aberrant morphologies including elongated and round multinucleated cells. This together with its localization at the mother-bud neck, suggest that Iqg1p promotes budding and cytokinesis. At restrictive temperatures, the vacuoles of the mutant cells enlarge and vesicles accumulate in the bud. Interestingly, Iqg1p shows two-hybrid interactions with the ankyrin repeat-containing protein, Akr1p (Kao, L.-R., J. Peterson, J. Ruiru, L. Bender, and A. Bender. 1996. Mol. Cell. Biol. 16:168-178), which inhibits pheromone signaling and appears to promote cytokinesis and/or trafficking. We also show two-hybrid interactions between Iqg1p and Afr1p, a septin-binding protein involved in projection formation (Konopka, J.B., C. DeMattei, and C. Davis. 1995. Mol. Cell. Biol. 15:723-730). We propose that Iqg1p acts as a scaffold to recruit and localize a protein complex involved in actin-based cellular functions and thus mediates the regulatory effects of Cdc42p on the actin cytoskeleton.
Figures












Similar articles
-
Identification of novel, evolutionarily conserved Cdc42p-interacting proteins and of redundant pathways linking Cdc24p and Cdc42p to actin polarization in yeast.Mol Biol Cell. 2000 Feb;11(2):773-93. doi: 10.1091/mbc.11.2.773. Mol Biol Cell. 2000. PMID: 10679030 Free PMC article.
-
The multiple roles of Cyk1p in the assembly and function of the actomyosin ring in budding yeast.Mol Biol Cell. 1999 Feb;10(2):283-96. doi: 10.1091/mbc.10.2.283. Mol Biol Cell. 1999. PMID: 9950677 Free PMC article.
-
The role of Cdc42p GTPase-activating proteins in assembly of the septin ring in yeast.Mol Biol Cell. 2003 Oct;14(10):4051-66. doi: 10.1091/mbc.e03-04-0247. Epub 2003 Jul 25. Mol Biol Cell. 2003. PMID: 14517318 Free PMC article.
-
Cdc42: An essential Rho-type GTPase controlling eukaryotic cell polarity.Microbiol Mol Biol Rev. 1999 Mar;63(1):54-105. doi: 10.1128/MMBR.63.1.54-105.1999. Microbiol Mol Biol Rev. 1999. PMID: 10066831 Free PMC article. Review.
-
Ras-related GTPases and the cytoskeleton.Mol Biol Cell. 1992 May;3(5):475-9. doi: 10.1091/mbc.3.5.475. Mol Biol Cell. 1992. PMID: 1611153 Free PMC article. Review.
Cited by
-
Cdk1-dependent control of membrane-trafficking dynamics.Mol Biol Cell. 2012 Sep;23(17):3336-47. doi: 10.1091/mbc.E11-10-0834. Epub 2012 Jul 5. Mol Biol Cell. 2012. PMID: 22767578 Free PMC article.
-
Saccharomyces cerevisiae cdc42p GTPase is involved in preventing the recurrence of bud emergence during the cell cycle.Mol Cell Biol. 2000 Nov;20(22):8548-59. doi: 10.1128/MCB.20.22.8548-8559.2000. Mol Cell Biol. 2000. PMID: 11046150 Free PMC article.
-
Timing it right: precise ON/OFF switches for Rho1 and Cdc42 GTPases in cytokinesis.J Cell Biol. 2013 Jul 22;202(2):187-9. doi: 10.1083/jcb.201306152. J Cell Biol. 2013. PMID: 23878271 Free PMC article.
-
A conserved role of IQGAP1 in regulating TOR complex 1.J Cell Sci. 2012 Apr 15;125(Pt 8):2041-52. doi: 10.1242/jcs.098947. Epub 2012 Feb 10. J Cell Sci. 2012. PMID: 22328503 Free PMC article.
-
CP110 cooperates with two calcium-binding proteins to regulate cytokinesis and genome stability.Mol Biol Cell. 2006 Aug;17(8):3423-34. doi: 10.1091/mbc.e06-04-0371. Epub 2006 Jun 7. Mol Biol Cell. 2006. PMID: 16760425 Free PMC article.
References
-
- Adams, A.E.M., and J.R. Pringle. 1991. Staining of actin with fluorochrome-conjugated phalloidin. Methods in Enzymology. 194:729–731. - PubMed
-
- Ausubel, F.M., R. Brent, R.E. Kingston, D.D. Moore, J.G. Seidman, J.A. Smith, and K. Struhl. 1992. Current Protocols in Molecular Biology. John Wiley and Sons Ltd., New York.
-
- Bagrodia S, Derijard B, Davis RJ, Cerione RA. Cdc42 and PAK-mediated signaling leads to jun kinase and p38 mitogen-activated protein kinase activation. J Biol Chem. 1995;270:27995–27998. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

