Cyclic nucleotide-gated channels on the flagellum control Ca2+ entry into sperm
- PMID: 9679145
- PMCID: PMC2133051
- DOI: 10.1083/jcb.142.2.473
Cyclic nucleotide-gated channels on the flagellum control Ca2+ entry into sperm
Abstract
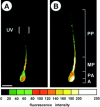
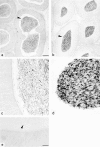
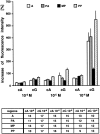
Cyclic nucleotide-gated (CNG) channels are key elements of cGMP- and cAMP-signaling pathways in vertebrate photoreceptor cells and in olfactory sensory neurons, respectively. These channels form heterooligomeric complexes composed of at least two distinct subunits (alpha and beta). The alpha subunit of cone photoreceptors is also present in mammalian sperm. Here we identify one short and several long less abundant transcripts of beta subunits in testis. The alpha and beta subunits are expressed in a characteristic temporal and spatial pattern in sperm and precursor cells. In mature sperm, the alpha subunit is observed along the entire flagellum, whereas the short beta subunit is restricted to the principal piece of the flagellum. These findings suggest that different forms of CNG channels coexist in the flagellum. Confocal microscopy in conjunction with the Ca2+ indicator Fluo-3 shows that the CNG channels serve as a Ca2+ entry pathway that responds more sensitively to cGMP than to cAMP. Assuming that CNG channel subtypes differ in their Ca2+ permeability, dissimilar localization of alpha and beta subunits may give rise to a pattern of Ca2+ microdomains along the flagellum, thereby providing the structural basis for control of flagellar bending waves.
Figures






References
-
- Ardell MD, Aragon I, Oliveira L, Porche GE, Burke E, Pittler SJ. The β subunit of human rod photoreceptor cGMP-gated cation channel is generated from a complex transcription unit. FEBS Lett. 1996;389:213–218. - PubMed
-
- Biel M, Zong X, Ludwig A, Sautter A, Hofmann F. Molecular cloning and expression of a modulatory subunit of the cyclic nucleotide-gated cation channel. J Biol Chem. 1996;271:6349–6355. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

