Integrin-mediated signals regulated by members of the rho family of GTPases
- PMID: 9679153
- PMCID: PMC2133065
- DOI: 10.1083/jcb.142.2.573
Integrin-mediated signals regulated by members of the rho family of GTPases
Abstract
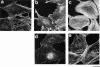
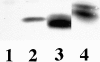
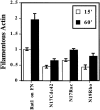
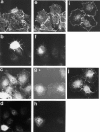
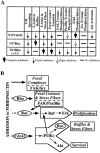
The organization of the actin cytoskeleton can be regulated by soluble factors that trigger signal transduction events involving the Rho family of GTPases. Since adhesive interactions are also capable of organizing the actin-based cytoskeleton, we examined the role of Cdc42-, Rac-, and Rho-dependent signaling pathways in regulating the cytoskeleton during integrin-mediated adhesion and cell spreading using dominant-inhibitory mutants of these GTPases. When Rat1 cells initially adhere to the extracellular matrix protein fibronectin, punctate focal complexes form at the cell periphery. Concomitant with focal complex formation, we observed some phosphorylation of the focal adhesion kinase (FAK) and Src, which occurred independently of Rho family GTPases. However, subsequent phosphorylation of FAK and paxillin occurs in a Rho-dependent manner. Moreover, we found Rho dependence of the assembly of large focal adhesions from which actin stress fibers radiate. Initial adhesion to fibronectin also stimulates membrane ruffling; we show that this ruffling is independent of Rho but is dependent on both Cdc42 and Rac. Furthermore, we observed that Cdc42 controls the integrin-dependent activation of extracellular signal-regulated kinase 2 and of Akt, a kinase whose activity has been demonstrated to be dependent on phosphatidylinositol (PI) 3-kinase. Since Rac-dependent membrane ruffling can be stimulated by PI 3-kinase, it appears that Cdc42, PI 3-kinase, and Rac lie on a distinct pathway that regulates adhesion-induced membrane ruffling. In contrast to the differential regulation of integrin-mediated signaling by Cdc42, Rac, and Rho, we observed that all three GTPases regulate cell spreading, an event that may indirectly control cellular architecture. Therefore, several separable signaling pathways regulated by different members of the Rho family of GTPases converge to control adhesion-dependent changes in the organization of the cytoskeleton, changes that regulate cell morphology and behavior.
Figures







References
-
- Aepfelbacher M, Essler M, Huber E, Czech A, Weber PC. Rho is a negative regulator of human monocyte spreading. J Immunol. 1996;157:5070–5075. - PubMed
-
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. - PubMed
-
- Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesion enhanced by Rho-kinase. Science. 1997;275:1308–1311. - PubMed
-
- Barry ST, Critchley DR. The RhoA-dependent assembly of focal adhesions in Swiss 3T3 cells is associated with increased tyrosine phosphorylation and the recruitment of both pp125FAK and protein kinase C-δ to focal adhesions. J Cell Sci. 1994;107:2033–2045. - PubMed
-
- Barry ST, Flinn HM, Humphries MJ, Critchley DR, Ridley AJ. Requirement for Rho in integrin signalling. Cell Adhes Commun. 1997;4:387–398. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

