The dependence of Ag+ block of a potassium channel, murine kir2.1, on a cysteine residue in the selectivity filter
- PMID: 9679159
- PMCID: PMC2231094
- DOI: 10.1111/j.1469-7793.1998.015bi.x
The dependence of Ag+ block of a potassium channel, murine kir2.1, on a cysteine residue in the selectivity filter
Abstract
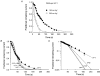
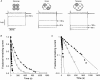
Externally applied Ag+ (100-200 nM) irreversibly blocked the strong inwardly rectifying K+ channel, Kir2.1. Mutation to serine of a cysteine residue at position 149 in the pore-forming H5 region of Kir2.1 abolished Ag+ blockage. To determine how many of the binding sites must be occupied by Ag+ before the channel is blocked, we measured the rate of channel block and found that our results were best fitted assuming that only one Ag+ ion need bind to eliminate channel current. We tested our hypothesis further by constructing covalently linked dimers and tetramers of Kir2.1 in which cysteine had been replaced by serine in one (dimer) or three (tetramer) of the linked subunits. When expressed, these constructs yielded functional channels with either two (dimer) or one (tetramer) cysteines per channel at position 149. Blockage in the tetramer was complete after sufficient exposure to 200 nM Ag+, a result that is also consistent with only one Ag+ being required to bind to Cys149 to block fully. The rate of development of blockage was 16 times slower than in wild-type channels; the rate was 4 times slower in channels formed from dimers.
Figures



References
-
- Akabas MH, Stauffer DA, Xu M, Karlin A. Acetylcholine receptor channel structure probed in cysteine-substitution mutants. Science. 1992;258:307–310. - PubMed
-
- Barry PH. JPCalc, a software package for calculating liquid junction potential corrections in patch clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. Journal of Neuroscience Methods. 1994;51:107–116. - PubMed
-
- Dance IG. Crystallization and structure of bis(tetramethylammonium) octakis(benzenethiolato)hexaargentate(I), extending the series of cages [M4(SPh)6]2−, [M5(SPh)7]2−, [M6(SPh)8]2−, and [M12(SPh)16]4−. Inorganic Chemistry. 1981;20:1487–1492.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

