Osmosensitive C1- currents and their relevance to regulatory volume decrease in human intestinal T84 cells: outwardly vs. inwardly rectifying currents
- PMID: 9679162
- PMCID: PMC2231111
- DOI: 10.1111/j.1469-7793.1998.045bi.x
Osmosensitive C1- currents and their relevance to regulatory volume decrease in human intestinal T84 cells: outwardly vs. inwardly rectifying currents
Abstract
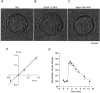
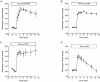
1. The swelling-activated outwardly rectifying Cl- current (ICl(swell)) recorded in T84 human intestinal cells was completely blocked by 10 microM tamoxifen, while 300 microM Cd2+ had no effect. 2. A ClC-2-like, inwardly rectifying Cl- current was activated after strong hyperpolarization in T84 cells. This current was completely inhibited by 300 microM Cd2+, unaffected by 10 microM tamoxifen, and its magnitude increased slightly in response to cell swelling under hyposmotic conditions. However, the swelling-dependent modulation occurred only after prior activation by hyperpolarizing voltages. 3. T84 cells behaved initially close to perfect osmometers in response to changes in external osmolalities between +20 and -30 %. The cells underwent full regulatory volume decrease (RVD) within 16 min when exposed to 30 or 10 % hyposmotic shocks. 4. Pharmacological tools were used to determine the anionic pathway(s) involved in RVD in T84 cells. Tamoxifen (10 microM), 1,9-dideoxyforskolin (DDFSK; 100 microM) and 4,4'-diisothiocyanatostilbene-2,2'-disulphonic acid (DIDS; 100 microM) blocked RVD while 300 microM Cd2+ had no effect upon RVD following a 30 % hyposmotic shock. The RVD response was similarly unaffected by Cd2+ when cells were exposed to a smaller (10 %) hyposmotic shock. 5. In conclusion, these data show that the anionic pathway primarily activated by cell swelling and relevant to RVD in T84 cells is the tamoxifen-, DDFSK- and DIDS-sensitive ICl(swell) and not the hyperpolarization-activated, Cd2+-sensitive Cl- current associated with the ClC-2 Cl- channel.
Figures
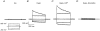
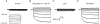
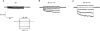
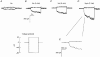

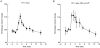
References
-
- Carew MA, Thorn P. Identification of ClC-2-like chloride currents in pig pancreatic acinar cells. Pflügers Archiv. 1996;433:84–90. - PubMed
-
- Chesnoy-Marchais D. Hyperpolarization-activated chloride channels in Aplysia neurons. In: Alvarez-Leefmans FJ, Russell JM, editors. Chloride Channels and Carriers in Nerve, Muscle and Glial Cells. New York: Plenum Press; 1990. pp. 367–382.
-
- Cid LP, Montrose-Rafizadeh C, Smith DI, Guggino WB, Cutting GR. Cloning of a putative human voltage-gated chloride channel (ClC-2) cDNA widely expressed in human tissues. Human Molecular Genetics. 1995;4:407–413. - PubMed
-
- Díaz M, Valverde MA, Higgins CF, Rucareanu C, Sepúlveda FV. Volume-activated chloride channels in HeLa cells are blocked by verapamil and dideoxyforskolin. Pflügers Archiv. 1993;422:347–353. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

