pH modulation of Ca2+ responses and a Ca2+-dependent K+ channel in cultured rat hippocampal neurones
- PMID: 9679168
- PMCID: PMC2231090
- DOI: 10.1111/j.1469-7793.1998.119bi.x
pH modulation of Ca2+ responses and a Ca2+-dependent K+ channel in cultured rat hippocampal neurones
Abstract
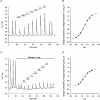
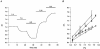
1. The effects of changes in extra- and intracellular pH (pHo and pHi, respectively) on depolarization-evoked rises in intracellular free Ca2+ concentration ([Ca2+]i) and the activity of a Ca2+-dependent K+ channel were investigated in cultured fetal rat hippocampal neurones. 2. In neurones loaded with 2', 7'-bis-(2-carboxyethyl)-5-(and -6)-carboxyfluorescein (BCECF), changes in pHo evoked changes in pHi. At room temperature, the ratio DeltapHi : DeltapHo (the slope of the regression line relating pHi to pHo) was 0.37 under HCO3-/CO2-buffered conditions and 0.45 under Hepes-buffered conditions; corresponding values at 37 C were 0.71 and 0.79, respectively. The measurements of changes in pHi evoked by changes in pHo were employed in subsequent experiments to correct for the effects of changes in pHi on the Kd of fura-2 for Ca2+. 3. In fura-2-loaded neurones, rises in [Ca2+]i evoked by transient exposure to 50 mM K+ were reduced and enhanced during perfusion with acidic and alkaline media, respectively, compared with control responses at pHo 7.3. Fifty percent inhibition of high-[K+]o-evoked rises in [Ca2+]i corresponded to pHo 7.23. In the presence of 10 microM nifedipine, 50 % inhibition of high-[K+]o-evoked responses corresponded to pHo 7.20, compared with a pHo of 7.31 for 50% inhibition of [Ca2+]i transients evoked by N-methyl-D-aspartate. 4. Changes in pHi at a constant pHo were evoked by exposing neurones to weak acids or bases and quantified in BCECF-loaded cells. Following pH-dependent corrections for the Kd of fura-2 for Ca2+, rises in [Ca2+]i evoked by high-[K+]o in fura-2-loaded cells were found to be affected only marginally by changes in pHi. When changes in pHi similar to those observed during the application of weak acids or bases were elicited by changing pHo, reductions in pH inhibited rises in [Ca2+]i evoked by 50 mM K+ whereas increases in pH enhanced them. 5. The effects of changes in pH on the kinetic properties of a BK-type Ca2+-dependent K+ channel were investigated. In inside-out patches excised from neurones in sister cultures to those used in the microspectrofluorimetric studies, with internal [Ca2+] at 20 microM, channel openings at an internal pH of 6.7 were generally absent whereas at pH 7.3 (or 7.8) the open probability was high. In contrast, channel activity in outside-out patches was not affected by reducing the pH of the bath (external) solution from 7.3 to 6.7. In inside-out patches with internal [Ca2+] at 0.7 microM, a separate protocol was applied to generate transient activation of the channel at a potential of 0 mV following a step from a holding level of -80 mV. In this case open probabilities were 0.81 (at pH 7.8), 0.57 (pH 7.3), 0.19 (pH 7.0) and 0.04 (pH 6.7). Channel conductance was not affected by changes in internal pH. 6. The results indicate that, in fetal rat hippocampal neurones, depolarization-evoked rises in [Ca2+]i mediated by the influx of Ca2+ ions through dihydropyridine-sensitive and -resistant voltage-activated Ca2+ channels are modulated by changes in pHo. The effects of pHo cannot be accounted for by changes in pHi consequent upon changes in pHo. However, changes in pHi affect the unitary properties of a Ca2+-dependent K+ channel. The results support the notion that pHo and/or pHi transients may serve a modulatory role in neuronal function.
Figures
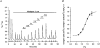




Similar articles
-
Effects of pH changes on calcium-mediated potentials in rat hippocampal neurons in vitro.Neuroscience. 1999 Mar;89(3):731-42. doi: 10.1016/s0306-4522(98)00344-3. Neuroscience. 1999. PMID: 10199608
-
Blockade by ifenprodil of high voltage-activated Ca2+ channels in rat and mouse cultured hippocampal pyramidal neurones: comparison with N-methyl-D-aspartate receptor antagonist actions.Br J Pharmacol. 1994 Oct;113(2):499-507. doi: 10.1111/j.1476-5381.1994.tb17017.x. Br J Pharmacol. 1994. PMID: 7834201 Free PMC article.
-
Anoxia-evoked intracellular pH and Ca2+ concentration changes in cultured postnatal rat hippocampal neurons.Neuroscience. 1999;93(3):1003-16. doi: 10.1016/s0306-4522(99)00230-4. Neuroscience. 1999. PMID: 10473265
-
Purification and characterization of epithelial Ca(2+)-activated K+ channels.Kidney Int. 1995 Oct;48(4):1047-56. doi: 10.1038/ki.1995.388. Kidney Int. 1995. PMID: 8569066 Review.
-
Magnesium homeostasis in cardiac cells.Mol Cell Biochem. 1992 Sep 8;114(1-2):97-103. doi: 10.1007/BF00240303. Mol Cell Biochem. 1992. PMID: 1461262 Review.
Cited by
-
GABAergic transmission and chloride equilibrium potential are not modulated by pyruvate in the developing optic tectum of Xenopus laevis tadpoles.PLoS One. 2012;7(4):e34446. doi: 10.1371/journal.pone.0034446. Epub 2012 Apr 4. PLoS One. 2012. PMID: 22496804 Free PMC article.
-
Stimulatory action of internal protons on Slo1 BK channels.Biophys J. 2003 May;84(5):2969-80. doi: 10.1016/S0006-3495(03)70023-X. Biophys J. 2003. PMID: 12719228 Free PMC article.
-
The influence of extracellular pH on the Ca2+ channels of the plasmalemma of Nitella syncarpa.Dokl Biochem Biophys. 2001 Nov-Dec;381:405-8. doi: 10.1023/a:1013367630645. Dokl Biochem Biophys. 2001. PMID: 11813555 No abstract available.
-
pH modulation of currents that contribute to the medium and slow afterhyperpolarizations in rat CA1 pyramidal neurones.J Physiol. 2004 Jan 15;554(Pt 2):449-66. doi: 10.1113/jphysiol.2003.051607. Epub 2003 Nov 7. J Physiol. 2004. PMID: 14608014 Free PMC article.
-
Topiramate hyperpolarizes and modulates the slow poststimulus AHP of rat olfactory cortical neurones in vitro.Br J Pharmacol. 2004 Jan;141(2):285-301. doi: 10.1038/sj.bjp.0705617. Epub 2003 Dec 22. Br J Pharmacol. 2004. PMID: 14691058 Free PMC article.
References
-
- Bevensee MO, Schwiening CJ, Boron WF. Use of BCECF and propidium iodide to assess membrane integrity of acutely isolated CA1 neurons from rat hippocampus. Journal of Neuroscience Methods. 1996;58:61–75. - PubMed
-
- Chesler M, Kaila K. Modulation of pH by neuronal activity. Trends in Neurosciences. 1992;15:396–402. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

