Functions of large conductance Ca2+-activated (BKCa), delayed rectifier (KV) and background K+ channels in the control of membrane potential in rabbit renal arcuate artery
- PMID: 9679171
- PMCID: PMC2231112
- DOI: 10.1111/j.1469-7793.1998.159bi.x
Functions of large conductance Ca2+-activated (BKCa), delayed rectifier (KV) and background K+ channels in the control of membrane potential in rabbit renal arcuate artery
Abstract
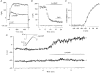
1. The types of K+ channel which determine the membrane potential of arcuate artery smooth muscle cells were investigated by patch-clamp recording from isolated cells and lumenal diameter measurements from intact pressurized renal arcuate arteries. 2. Single cells had a mean resting potential of -38 mV and were depolarized by 130 mM K+ but not by the Cl- channel blocker 4,4'-diisothiocyanatostilbene-2, 2'-disulphonic acid (DIDS). 3. Iberiotoxin did not affect the resting potential but inhibited spontaneous transient hyperpolarizations. Iberiotoxin or 1 mM tetraethylammonium (TEA+) constricted intact arteries. 3,4-Diaminopyridine (3,4-DAP)-sensitive delayed rectifier K+ (KV) channel current was elicited by depolarization but 3,4-DAP did not affect the resting potential or induce constriction in the intact artery. 4. A voltage-independent K+ current was inhibited by >= 0.1 mM barium (Ba2+) and unaffected by iberiotoxin, glibenclamide, apamin, 3,4-DAP and ouabain. In six out of ten cells, 1 mM Ba2+ depolarized the resting potential, while in the other cells the potential was resistant to all of the K+ channel blockers and ouabain. Ba2+ (0.1-1 mM) constricted the intact artery, but 10 microM Ba2+, 1 microM glibenclamide or 100 nM apamin had no effect. 5. The data suggest that resting potential is determined by background K+ channels, one type being Ba2+ sensitive and voltage independent, and another type being poorly defined due to its resistance to any inhibitor. Large conductance Ca2+-activated K+ (BKCa) and KV channels do not determine the resting potential but have separate functions to underlie transient Ca2+-induced hyperpolarizations and to protect against depolarization past about -30 mV.
Figures
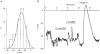




References
-
- Archer SL, Huang JMC, Reeve HL, Hampl V, Tolarová S, Michelakis E, Weir EK. Differential distribution of electrophysiologically distinct myocytes in conduit and resistance arteries determines their response to nitric oxide and hypoxia. Circulation Research. 1996;78:431–442. - PubMed
-
- Bolton TB, Imaizumi Y. Spontaneous transient outward currents in smooth muscle cells. Cell Calcium. 1996;20:141–152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

