Does a C3-C4 propriospinal system transmit corticospinal excitation in the primate? An investigation in the macaque monkey
- PMID: 9679174
- PMCID: PMC2231097
- DOI: 10.1111/j.1469-7793.1998.191bi.x
Does a C3-C4 propriospinal system transmit corticospinal excitation in the primate? An investigation in the macaque monkey
Abstract
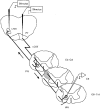
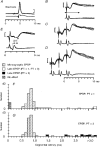
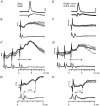
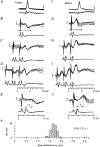
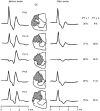
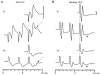
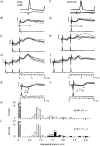
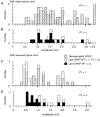
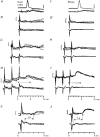
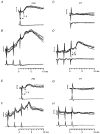
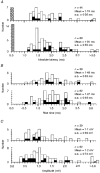
1. Synaptic responses to electrical stimulation of the contralateral pyramidal tract were measured in intracellular recordings from 206 upper limb motoneurones in ten chloralose-anaesthetized macaque monkeys. The objective was to search for evidence of a disynaptic excitatory pathway via C3-C4 propriospinal interneurones similar to that in the cat. 2. In monkeys with intact spinal cords, only a small proportion of motoneurones (19%) responded with late EPSPs to repetitive stimulation of the pyramid; only 3% had segmental latencies that were appropriate for a disynaptic pathway. 3. From previous studies in the cat, it was expected that a lesion to the dorsolateral funiculus (DLF) at C5 would interrupt the corticospinal input to the spinal segments supplying upper limb muscles, whilst leaving intact excitation transmitted via a C3-C4 propriospinal system, the descending axons of which travel in the ventral part of the funiculus. In five of the monkeys a lesion was made to the DLF at C5 which spared the ventrolateral columns. It severely reduced the monosynaptic EPSPs and disynaptic IPSPs evoked from the pyramidal tract that were present in the intact monkey spinal cord, and which might have masked the presence of disynaptic EPSPs. However, even after the lesion the proportion of motoneurones with such late EPSPs was still low (18%); 14% of motoneurones had EPSPs within the disynaptic range. 4. In addition, some EPSPs with relatively long segmental latencies (> 1.1 ms) were recorded before and after the C5 lesions, but since these effects could be evoked by single stimuli, had stable latencies and did not facilitate with repetitive shocks, it is likely that they represent monosynaptic EPSPs evoked by slowly conducting corticospinal fibres which survived the lesions. 5. In seven of the monkeys motoneurone responses to stimulation of the ipsilateral lateral reticular nucleus (LRN) were also tested. Most motoneurones showed EPSPs with short latencies (1.2-2.5 ms) and other properties characteristic of monosynaptic activation. This is consistent with the presence of collaterals of C3-C4 propriospinal neurones to the LRN, as demonstrated in the cat. 6. These short-latency EPSPs evoked from the LRN were just as common before (77%) as after (75%) the C5 lesion. They had small amplitudes both before (mean +/- s.d. 1.1 +/- 0.59 mV) and after (1.2 +/- 0.72 mV) the lesion. Unlike the situation in the cat, only a small proportion (16%) of motoneurones activated from the LRN showed late EPSPs after repetitive stimulation of the pyramid. 7. The results provide little evidence for significant corticospinal excitation of motoneurones via a system of C3-C4 propriospinal neurones in the monkey. The general absence of responses mediated by such a system in the macaque, under experimental conditions similar to those in which they are seen in the cat, show that extrapolation of results from the cat to the primate should be made with considerable caution.
Figures


Similar articles
-
Properties of propriospinal neurons in the C3-C4 segments mediating disynaptic pyramidal excitation to forelimb motoneurons in the macaque monkey.J Neurophysiol. 2006 Jun;95(6):3674-85. doi: 10.1152/jn.00103.2005. Epub 2006 Feb 22. J Neurophysiol. 2006. PMID: 16495365
-
Striking differences in transmission of corticospinal excitation to upper limb motoneurons in two primate species.J Neurophysiol. 2000 Aug;84(2):698-709. doi: 10.1152/jn.2000.84.2.698. J Neurophysiol. 2000. PMID: 10938297
-
Integration in descending motor pathways controlling the forelimb in the cat. 10. Inhibitory pathways to forelimb motoneurones via C3-C4 propriospinal neurones.Exp Brain Res. 1984;56(2):279-92. doi: 10.1007/BF00236284. Exp Brain Res. 1984. PMID: 6090195
-
Spinal interneuronal networks in the cat: elementary components.Brain Res Rev. 2008 Jan;57(1):46-55. doi: 10.1016/j.brainresrev.2007.06.022. Epub 2007 Aug 6. Brain Res Rev. 2008. PMID: 17884173 Free PMC article. Review.
-
The Corticospinal System and Amyotrophic Lateral Sclerosis: IFCN handbook chapter.Clin Neurophysiol. 2024 Apr;160:56-67. doi: 10.1016/j.clinph.2024.02.001. Epub 2024 Feb 9. Clin Neurophysiol. 2024. PMID: 38401191 Review.
Cited by
-
Responses of single corticospinal neurons to intracortical stimulation of primary motor and premotor cortex in the anesthetized macaque monkey.J Neurophysiol. 2013 Jun;109(12):2982-98. doi: 10.1152/jn.01080.2012. Epub 2013 Mar 27. J Neurophysiol. 2013. PMID: 23536718 Free PMC article.
-
Axon diameters and conduction velocities in the macaque pyramidal tract.J Neurophysiol. 2014 Sep 15;112(6):1229-40. doi: 10.1152/jn.00720.2013. Epub 2014 May 28. J Neurophysiol. 2014. PMID: 24872533 Free PMC article.
-
Connected corticospinal sites show enhanced tuning similarity at the onset of voluntary action.J Neurosci. 2007 Nov 7;27(45):12349-57. doi: 10.1523/JNEUROSCI.3127-07.2007. J Neurosci. 2007. PMID: 17989299 Free PMC article.
-
The effect of electrical stimulation of the corticospinal tract on motor units of the human biceps brachii.J Physiol. 2002 Oct 1;544(Pt 1):277-84. doi: 10.1113/jphysiol.2002.024539. J Physiol. 2002. PMID: 12356898 Free PMC article.
-
Cortical presynaptic control of dorsal horn C-afferents in the rat.PLoS One. 2013 Jul 30;8(7):e69063. doi: 10.1371/journal.pone.0069063. Print 2013. PLoS One. 2013. PMID: 23935924 Free PMC article.
References
-
- Alstermark B, Kümmel H, Pinter MJ, Tantisira B. Integration in descending motor pathways controlling the forelimb in the cat. 17. Axonal projection and termination of C3-C4 propriospinal neurones in the C6-Th1 segments. Experimental Brain Research. 1990;81:447–461. - PubMed
-
- Alstermark B, Lindström S, Lundberg A, Sybirska E. Integration in descending motor pathways controlling the forelimb of the cat. 8. Ascending projection to the lateral reticular nucleus from C3-C4 propriospinal neurones also projecting to forelimb motoneurones. Experimental Brain Research. 1981a;42:282–298. - PubMed
-
- Alstermark B, Lundberg A. The C3-C4 propriospinal system: target reaching and food-taking. In: Jami L, Pierrot-Deseilligny E, Zytnicki D, editors. Muscle Afferents and Spinal Control of Movement. London: Pergamon Press; 1992. pp. 327–354.
-
- Alstermark B, Lundberg A, Norrsell U, Sybirska E. Integration in descending motor pathways controlling the forelimb in the cat. 9. Differential behavioural defects after spinal cord lesions interrupting defined pathways from higher centres to motoneurones. Experimental Brain Research. 1981b;42:299–318. - PubMed
-
- Alstermark B, Lundberg A, Sasaki S. Integration in descending motor pathways controlling the forelimb in the cat. 12. Interneurones which may mediate descending feed-forward inhibition and feed-back inhibition from the forelimb to C3-C4 propriospinal neurones. Experimental Brain Research. 1984;56:308–322. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

