Delayed production of adenosine underlies temporal modulation of swimming in frog embryo
- PMID: 9679180
- PMCID: PMC2231108
- DOI: 10.1111/j.1469-7793.1998.265bi.x
Delayed production of adenosine underlies temporal modulation of swimming in frog embryo
Abstract
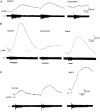
1. To investigate the dynamics of adenosine production in the spinal cord during motor activity, and its possible contribution to the temporal modulation of motor patterns, a sensor sensitive to adenosine at concentrations as low as 10 nM was devised. 2. When pressed against the outside of the spinal cord, the sensor detected slow changes in the levels of adenosine during fictive swimming that ranged from 10 to 650 nM. In four embryos where particularly large signals were recorded due to favourable probe placement, the adenosine levels continued to rise for up to a minute following cessation of activity before slowly returning to baseline. In the remaining thirteen embryos, levels of adenosine started to return slowly to baseline almost immediately after activity had stopped. 3. Inhibitors of adenosine uptake increased the magnitude of the signal recorded and slowed the recovery following cessation of activity. 4. A realistic computational model of the spinal circuitry was combined with models of extracellular breakdown of ATP to adenosine. ATP and adenosine inhibited, as in the real embryo, the voltage-gated K+ and Ca2+ currents, respectively. The model reproduced the temporal run-down of motor activity seen in the real embryo suggesting that synaptic release of ATP together with its extracellular breakdown to adenosine is sufficient to exert time-dependent control over motor pattern generation. 5. The computational analysis also suggested that the delay in the rise of adenosine levels is likely to result from feed-forward inhibition of the 5'-ectonucleotidase in the spinal cord. This inhibition is a key determinant of the rate of run-down.
Figures


References
-
- Agarwal RP, Parks RE. Adenosine deaminase from human erythrocytes. Methods in Enzymology. 1978;51:502–507. - PubMed
-
- Arshavsky YI, Orlovsky GN, Panchin YV, Roberts A, Soffe SR. Neuronal control of swimming locomotion: analysis of the pteropod mollusc Clione and embryos of the amphibian Xenopus. Trends in Neurosciences. 1993;16:227–232. 10.1016/0166-2236(93)90161-E. - DOI - PubMed
-
- Berners MOM, Boutelle MG, Fillenz M. Online measurement of brain glutamate with an enzyme/polymer coated tubular electrode. Analytical Chemistry. 1994;66:2017–2021. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

