YscO of Yersinia pestis is a mobile core component of the Yop secretion system
- PMID: 9683485
- PMCID: PMC107372
- DOI: 10.1128/JB.180.15.3882-3890.1998
YscO of Yersinia pestis is a mobile core component of the Yop secretion system
Abstract
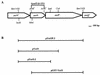
The Yersinia pestis low-Ca2+ response stimulon is responsible for the temperature- and Ca2+-regulated expression and secretion of plasmid pCD1-encoded antihost proteins (V antigen and Yops). We have previously shown that lcrD, yscC, yscD, yscG, and yscR encode proteins that are essential for high-level expression and secretion of V antigen and Yops at 37 degreesC in the absence of Ca2+. In this study, we characterized yscO of the Yop secretion (ysc) operon that contains yscN through yscU by determining the localization of its gene product and the phenotype of an in-frame deletion. The yscO mutant grew and expressed the same levels of Yops as the parent at 37 degreesC in the presence of Ca2+. In the absence of Ca2+, the mutant grew independently of Ca2+, expressed only basal levels of V antigen and Yops, and failed to secrete these. These defects could be partially complemented by providing yscO in trans in the yscO mutant. Overexpression of YopM and V antigen in the mutant failed to restore the export of either protein, showing that the mutation had a direct effect on secretion. These results indicated that the yscO gene product is required for high-level expression and secretion of V antigen and Yops. YscO was found by immunoblot analysis in the soluble and membrane fractions of bacteria growing at 37 degreesC irrespective of the presence of Ca2+ and in the culture medium in the absence of Ca2+. YscO is the only mobile protein identified so far in the Yersinia species that is required for secretion of V antigen and Yops.
Figures


Similar articles
-
Mutations in yscC, yscD, and yscG prevent high-level expression and secretion of V antigen and Yops in Yersinia pestis.J Bacteriol. 1995 Jul;177(13):3843-54. doi: 10.1128/jb.177.13.3843-3854.1995. J Bacteriol. 1995. PMID: 7601852 Free PMC article.
-
YscP of Yersinia pestis is a secreted component of the Yop secretion system.J Bacteriol. 1999 May;181(9):2852-62. doi: 10.1128/JB.181.9.2852-2862.1999. J Bacteriol. 1999. PMID: 10217778 Free PMC article.
-
The V antigen of Yersinia pestis regulates Yop vectorial targeting as well as Yop secretion through effects on YopB and LcrG.J Bacteriol. 1998 Jul;180(13):3410-20. doi: 10.1128/JB.180.13.3410-3420.1998. J Bacteriol. 1998. PMID: 9642196 Free PMC article.
-
The virulence plasmid of Yersinia, an antihost genome.Microbiol Mol Biol Rev. 1998 Dec;62(4):1315-52. doi: 10.1128/MMBR.62.4.1315-1352.1998. Microbiol Mol Biol Rev. 1998. PMID: 9841674 Free PMC article. Review.
-
Roles of YopN, LcrG and LcrV in controlling Yops secretion by Yersinia pestis.Adv Exp Med Biol. 2007;603:225-34. doi: 10.1007/978-0-387-72124-8_20. Adv Exp Med Biol. 2007. PMID: 17966419 Review.
Cited by
-
Impassable YscP substrates and their impact on the Yersinia enterocolitica type III secretion pathway.J Bacteriol. 2008 Sep;190(18):6204-16. doi: 10.1128/JB.00467-08. Epub 2008 Jul 18. J Bacteriol. 2008. PMID: 18641141 Free PMC article.
-
Selective binding of virulence type III export chaperones by FliJ escort orthologues InvI and YscO.FEMS Microbiol Lett. 2009 Apr;293(2):292-7. doi: 10.1111/j.1574-6968.2009.01535.x. Epub 2009 Mar 2. FEMS Microbiol Lett. 2009. PMID: 19260965 Free PMC article.
-
Orf1B controls secretion of T3SS proteins and contributes to Edwardsiella piscicida adhesion to epithelial cells.Vet Res. 2022 Jun 13;53(1):40. doi: 10.1186/s13567-022-01057-6. Vet Res. 2022. PMID: 35692056 Free PMC article.
-
DNA sequencing and analysis of the low-Ca2+-response plasmid pCD1 of Yersinia pestis KIM5.Infect Immun. 1998 Oct;66(10):4611-23. doi: 10.1128/IAI.66.10.4611-4623.1998. Infect Immun. 1998. PMID: 9746557 Free PMC article.
-
Common architecture of the flagellar type III protein export apparatus and F- and V-type ATPases.Nat Struct Mol Biol. 2011 Mar;18(3):277-82. doi: 10.1038/nsmb.1977. Epub 2011 Jan 30. Nat Struct Mol Biol. 2011. PMID: 21278755
References
-
- Allaoui A, Schulte R, Cornelis G R. Mutational analysis of the Yersinia enterocolitica virC operon: characterization of yscE, F, G, I, J, K required for Yop secretion and yscHencoding YopR. Mol Microbiol. 1995;18:343–355. - PubMed
-
- Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Anderson D M, Schneewind O. A mRNA signal for the type III secretion of Yop proteins by Yersinia enterocolitica. Science. 1997;278:1140–1143. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

