Regulation of porin-mediated outer membrane permeability by nutrient limitation in Escherichia coli
- PMID: 9683489
- PMCID: PMC107376
- DOI: 10.1128/JB.180.15.3917-3922.1998
Regulation of porin-mediated outer membrane permeability by nutrient limitation in Escherichia coli
Abstract
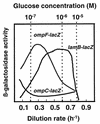
OmpF and OmpC porins were differentially regulated by nutrient limitation and growth rate in glucose- or nitrogen-limited chemostat cultures of Escherichia coli. Transcriptional and translational ompF fusions showed a sharp peak of expression under glucose limitation at D = 0.3 h-1, with lower amounts at lower and higher growth rates. The peak of OmpR-dependent transcriptional stimulation of ompF under glucose limitation in minimal salts media was about 20-fold above nutrient excess levels and 3-fold higher than that achieved with low osmolarity. Analysis of outer membrane protein levels and results of growth competition experiments with porin mutants were consistent with the enhanced role of OmpF under glucose limitation, but not N limitation. In contrast, OmpC was the major porin under N limitation but was increasingly expressed under glucose limitation at very low growth rates approaching starvation, when OmpF was downregulated. In summary, outer membrane permeability under N-limited, sugar-rich conditions is largely based on OmpC, whereas porin activity is a complex, highly sensitive function of OmpF, OmpC, and LamB glycoporin expression under different levels of glucose limitation. Indeed, the OmpF level was more responsive to nutrient limitation than to medium osmolarity and suggested a significant additional layer of control over the porin-regulatory network.
Figures
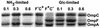


References
-
- Andersen J, Delihas N. micF RNA binds to the 5′ end of ompF mRNA and to a protein from Escherichia coli. Biochemistry. 1990;29:9249–9256. - PubMed
-
- Ferenci T. Adaptation to life at micromolar nutrient levels: the regulation of Escherichia coli glucose transport by endoinduction and cAMP. FEMS Microbiol Rev. 1996;18:301–317. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

