Bacterial SOS checkpoint protein SulA inhibits polymerization of purified FtsZ cell division protein
- PMID: 9683493
- PMCID: PMC107380
- DOI: 10.1128/JB.180.15.3946-3953.1998
Bacterial SOS checkpoint protein SulA inhibits polymerization of purified FtsZ cell division protein
Abstract
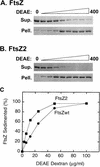
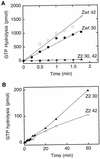
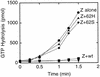
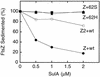
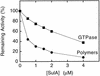
Cell division of Escherichia coli is inhibited when the SulA protein is induced in response to DNA damage as part of the SOS checkpoint control system. The SulA protein interacts with the tubulin-like FtsZ division protein. We investigated the effects of purified SulA upon FtsZ. SulA protein inhibits the polymerization and the GTPase activity of FtsZ, while point mutant SulA proteins show little effect on either of these FtsZ activities. SulA did not inhibit the polymerization of purified FtsZ2 mutant protein, which was originally isolated as insensitive to SulA. These studies define polymerization assays for FtsZ which respond to an authentic cellular regulator. The observations presented here support the notion that polymerization of FtsZ is central to its cellular role and that direct, reversible inhibition of FtsZ polymerization by SulA may account for division inhibition.
Figures



References
-
- Bi E F, Lutkenhaus J. FtsZ ring structure associated with division in Escherichia coli. Nature. 1991;354:161–164. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

