Effect of sensory disuse on geniculate afferents to cat visual cortex
- PMID: 9685193
- PMCID: PMC2430473
- DOI: 10.1017/s0952523898153105
Effect of sensory disuse on geniculate afferents to cat visual cortex
Abstract
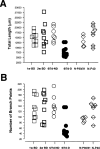
In the kitten, as little as a week of monocular lid suture during early life causes a remarkable remodeling of the geniculocortical projections serving the deprived eye (Antonini & Stryker, 1993a, 1996). While the physiological effects of monocular deprivation have been shown to be due to competitive interactions between the projections serving the two eyes, it is not known whether these morphological changes are due to competitive interactions or to sensory disuse. We addressed this question by analyzing the morphology of geniculocortical arbors in kittens deprived of patterned vision by binocular lid suture for 1 week or 2 weeks ending at 6 weeks of age. Such deprivation would be expected to affect the afferents serving the two eyes equally, giving neither eye a competitive advantage. The arbors were anterogradely filled with Phaseolus lectin iontophoretically injected into lamina A of the lateral geniculate nucleus. The lectin was visualized immunohistochemically, and single geniculocortical arbors were serially reconstructed in three dimensions. Arbors reconstructed in binocularly deprived animals were compared with arbors serving the deprived and nondeprived eye in animals monocularly deprived by lid suture of one eye for a week and with arbors obtained in age-matched normal controls. Geniculocortical arbors in binocularly deprived animals did not suffer the drastic remodeling of the deprived arbors in monocularly deprived animals. Indeed, arbors in binocularly deprived animals were indistinguishable from arbors in normal kittens or nondeprived arbors in short-term monocularly deprived animals. These results support the notion that competitive mechanisms rather than sensory disuse are responsible for gross morphological remodeling of geniculocortical arbors.
Figures




References
-
- Antonini A, Stryker MP. Rapid remodeling of axonal arbors in the visual cortex. Science. 1993a;260:1819–1821. - PubMed
-
- Antonini A, Stryker MP. Geniculocortical afferent arbors in binocularly deprived kittens. Society for Neuroscience Abstracts. 1995;21:795.3, 2023.
-
- Antonini A, Stryker MP. Plasticity of geniculocortical afferents following brief or prolonged monocular occlusion in the cat. Journal of Comparative Neurology. 1996;369:64–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

