Efficacy of nitazoxanide against Cryptosporidium parvum in cell culture and in animal models
- PMID: 9687390
- PMCID: PMC105716
- DOI: 10.1128/AAC.42.8.1959
Efficacy of nitazoxanide against Cryptosporidium parvum in cell culture and in animal models
Abstract
Nitazoxanide (NTZ), a drug currently being tested in human clinical trials for efficacy against chronic cryptosporidiosis, was assessed in cell culture and in two animal models. The inhibitory activity of NTZ was compared with that of paromomycin (PRM), a drug that is partially effective against Cryptosporidium parvum. A concentration of 10 microg of NTZ/ml (32 microM) consistently reduced parasite growth in cell culture by more than 90% with little evidence of drug-associated cytotoxicity, in contrast to an 80% reduction produced by PRM at 2,000 microg/ml (3.2 mM). In contrast to its efficacy in vitro, NTZ at either 100 or 200 mg/kg of body weight/day for 10 days was ineffective at reducing the parasite burden in C. parvum-infected, anti-gamma-interferon-conditioned SCID mice. Combined treatment with NTZ and PRM was no more effective than treatment with PRM alone. Finally, NTZ was partially effective at reducing the parasite burden in a gnotobiotic piglet diarrhea model when given orally for 11 days at 250 mg/kg/day but not at 125 mg/kg/day. However, the higher dose of NTZ induced a drug-related diarrhea in piglets that might have influenced its therapeutic efficacy. As we have previously reported, PRM was effective at markedly reducing the parasite burden in piglets at a dosage of 500 mg/kg/day. Our results indicate that of all of the models tested, the piglet diarrhea model most closely mimics the partial response to NTZ treatment reported to occur in patients with chronic cryptosporidiosis.
Figures

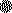 , 100 mg of NTZ/kg/day and 2,500 mg of PRM/kg/day; ▾, 200
mg of NTZ/kg/day and 2,500 mg of PRM/kg/day; ▴, 2,500 mg of
PRM/kg/day;
, 100 mg of NTZ/kg/day and 2,500 mg of PRM/kg/day; ▾, 200
mg of NTZ/kg/day and 2,500 mg of PRM/kg/day; ▴, 2,500 mg of
PRM/kg/day;  , 100 mg of NTZ/kg/day; □, 200 mg of NTZ/kg/day; and
◊, placebo.
, 100 mg of NTZ/kg/day; □, 200 mg of NTZ/kg/day; and
◊, placebo.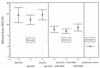
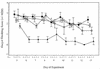
 ) or 250 (◊) mg/kg/day, placebo
(■), or PRM at 500 mg/kg/day (▴). In multiple regression analysis,
the oocyst excretion score was found to be significantly related to
treatment group, with the highest scores being observed in the placebo
group, followed by the lower-dose NTZ group and then the higher-dose
NTZ group, and the lowest scores being observed in the PRM group
(F = 42.507; P < 0.001). Further comparison
revealed that, during days 7 through 13, the scores for the piglets
treated with NTZ at 250 mg/kg/day were significantly lower than those
for the placebo-treated piglets (Z = −3.258;
P = 0.001, two-tailed Wilcoxon signed rank test). In
contrast, subgroup comparison of the infected placebo-treated control
piglets and piglets treated with NTZ at 125 mg/kg/day did not reveal
any significant difference. Oocyst shedding was significantly less
marked in the PRM-treated group than in any of the other groups
(P < 0.001). Values are means ± standard errors of
the means (SEM).
) or 250 (◊) mg/kg/day, placebo
(■), or PRM at 500 mg/kg/day (▴). In multiple regression analysis,
the oocyst excretion score was found to be significantly related to
treatment group, with the highest scores being observed in the placebo
group, followed by the lower-dose NTZ group and then the higher-dose
NTZ group, and the lowest scores being observed in the PRM group
(F = 42.507; P < 0.001). Further comparison
revealed that, during days 7 through 13, the scores for the piglets
treated with NTZ at 250 mg/kg/day were significantly lower than those
for the placebo-treated piglets (Z = −3.258;
P = 0.001, two-tailed Wilcoxon signed rank test). In
contrast, subgroup comparison of the infected placebo-treated control
piglets and piglets treated with NTZ at 125 mg/kg/day did not reveal
any significant difference. Oocyst shedding was significantly less
marked in the PRM-treated group than in any of the other groups
(P < 0.001). Values are means ± standard errors of
the means (SEM).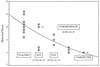
Similar articles
-
The immunomodulatory activity of secnidazole-nitazoxanide in a murine cryptosporidiosis model.J Med Microbiol. 2021 Mar;70(3). doi: 10.1099/jmm.0.001327. Epub 2021 Feb 23. J Med Microbiol. 2021. PMID: 33625354
-
Long-lasting anticryptosporidial activity of nitazoxanide in an immunosuppressed rat model.Folia Parasitol (Praha). 2003 Mar;50(1):19-22. doi: 10.14411/fp.2003.003. Folia Parasitol (Praha). 2003. PMID: 12735719
-
Nitazoxanide in the treatment of cryptosporidial diarrhea and other intestinal parasitic infections associated with acquired immunodeficiency syndrome in tropical Africa.Am J Trop Med Hyg. 1997 Jun;56(6):637-9. doi: 10.4269/ajtmh.1997.56.637. Am J Trop Med Hyg. 1997. PMID: 9230795 Clinical Trial.
-
Drug treatment and novel drug target against Cryptosporidium.Parasite. 2008 Sep;15(3):275-81. doi: 10.1051/parasite/2008153275. Parasite. 2008. PMID: 18814694 Review.
-
Cryptosporidiosis Drug Discovery: Opportunities and Challenges.ACS Infect Dis. 2016 Aug 12;2(8):530-7. doi: 10.1021/acsinfecdis.6b00094. Epub 2016 Jul 22. ACS Infect Dis. 2016. PMID: 27626293 Review.
Cited by
-
Exploring the therapeutic potential of mitochondrial uncouplers in cancer.Mol Metab. 2021 Sep;51:101222. doi: 10.1016/j.molmet.2021.101222. Epub 2021 Mar 26. Mol Metab. 2021. PMID: 33781939 Free PMC article. Review.
-
Repositioning of Anthelmintic Drugs for the Treatment of Cancers of the Digestive System.Int J Mol Sci. 2020 Jul 13;21(14):4957. doi: 10.3390/ijms21144957. Int J Mol Sci. 2020. PMID: 32668817 Free PMC article. Review.
-
Functional expression of a DNA-topoisomerase IB from Cryptosporidium parvum.J Biomed Biotechnol. 2009;2009:837608. doi: 10.1155/2009/837608. Epub 2009 Jul 27. J Biomed Biotechnol. 2009. PMID: 19644560 Free PMC article.
-
Cryptosporidium parvum DNA replication in cell-free culture.J Parasitol. 2009 Oct;95(5):1239-42. doi: 10.1645/GE-2052.1. Epub 2009 May 23. J Parasitol. 2009. PMID: 19463037 Free PMC article.
-
A Cryptosporidium PI(4)K inhibitor is a drug candidate for cryptosporidiosis.Nature. 2017 Jun 15;546(7658):376-380. doi: 10.1038/nature22337. Epub 2017 May 31. Nature. 2017. PMID: 28562588 Free PMC article.
References
-
- Blagburn B L, Soave R. Prophylaxis and chemotherapy: human and animal. In: Fayer R, editor. Cryptosporidium and cryptosporidiosis—1997. Boca Raton, Fla: CRC Press; 1997. pp. 111–128.
-
- Cavier R, Rossignol J F. Pharmacological study of various antihelminthic combinations. Rev Med Vet. 1982;133:779–783.
-
- Current W L. Techniques and laboratory maintenance of Cryptosporidium. In: Pubey J P, Speer C A, Fayer R, editors. Cryptosporidiosis of man and animals. Boston, Mass: CRC Press; 1990. pp. 31–49.
-
- Davis L J, Soave R, Dudley R E, Fessel J W, Faulkner S, Mamakos J P. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Nitazoxanide (NTZ) for AIDS-related cryptosporidial diarrhea (CD): an open-label safety, efficacy and pharmacokinetic study, abstr. LM50; p. 289.
-
- Doumbo O, Rossignol J F, Pichard E, et al. Nitazoxanide in treatment of cryptosporidial diarrhea and other intestinal parasitic infections associated with acquired immunodeficiency syndrome in tropical Africa. Am J Trop Med Hyg. 1997;56:637–639. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

