Purification and characterization of a high-molecular-weight insecticidal protein complex produced by the entomopathogenic bacterium photorhabdus luminescens
- PMID: 9687469
- PMCID: PMC106811
- DOI: 10.1128/AEM.64.8.3029-3035.1998
Purification and characterization of a high-molecular-weight insecticidal protein complex produced by the entomopathogenic bacterium photorhabdus luminescens
Abstract
Photorhabdus luminescens is a gram-negative enteric bacterium that is found in association with entomopathogenic nematodes of the family Heterorhabditidae. The nematodes infect a variety of soil-dwelling insects. Upon entering an insect host, the nematode releases P. luminescens cells from its intestinal tract, and the bacteria quickly establish a lethal septicemia. When grown in peptone broth, in the absence of the nematodes, the bacteria produce a protein toxin complex that is lethal when fed to, or injected into the hemolymph of, Manduca sexta larvae and several other insect species. The toxin purified as a protein complex which has an estimated molecular weight of 1,000,000 and contains no protease, phospholipase, or hemolytic activity and only a trace of lipase activity. The purified toxin possesses insecticidal activity whether injected or given orally. Analyses of the denatured complex by sodium dodecyl sulfate-polyacrylamide gel electrophoresis showed it to be composed of several protein subunits ranging in size from 30 to 200 kDa. The complex was further separated by native gel electrophoresis into three components, two of which retained insecticidal activity. The purified native toxin complex was found to be active in nanogram concentrations against insects representing four orders of the class Insecta.
Figures
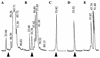
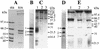
References
-
- Akhurst R J. Morphological and functional dimorphism in Xenorhabdus spp. bacteria symbiotically associated with the insect pathogenic nematodes Neoaplectana and Heterorhabditis. J Gen Microbiol. 1980;121:303–309.
-
- Akhurst R J. Antibiotic activity of Xenorhabdus spp., bacteria symbiotically associated with insect pathogenic nematodes of the families Heterorhabditidae and Steinernematidae. J Gen Microbiol. 1982;128:3061–3065. - PubMed
-
- Bedding R, Akhurst R, Kaya H. Nematodes and their biological control of insect pests. Melbourne, Australia: CSIRO Publications; 1993. pp. 1–178.
LinkOut - more resources
Full Text Sources
Other Literature Sources

