Trophoblast class I major histocompatibility complex (MHC) products are resistant to rapid degradation imposed by the human cytomegalovirus (HCMV) gene products US2 and US11
- PMID: 9687527
- PMCID: PMC2212475
- DOI: 10.1084/jem.188.3.497
Trophoblast class I major histocompatibility complex (MHC) products are resistant to rapid degradation imposed by the human cytomegalovirus (HCMV) gene products US2 and US11
Abstract
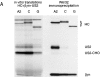
US11 and US2 encode gene products expressed early in the replicative cycle of human cytomegalovirus (HCMV), which cause dislocation of human and murine major histocompatibility complex (MHC) class I molecules from the lumen of the endoplasmic reticulum to the cytosol, where the class I heavy chains are rapidly degraded. Human histocompatibility leukocyte antigens (HLA)-C and HLA-G are uniquely resistant to the effects of both US11 and US2 in a human trophoblast cell line as well as in porcine endothelial cells stably transfected with human class I genes. Dislocation and degradation of MHC class I heavy chains do not appear to involve cell type-specific factors, as US11 and US2 are fully active in this xenogeneic model. Importantly, trophoblasts HLA-G and HLA-C possess unique characteristics that allow their escape from HCMV-associated MHC class I degradation. Trophoblast class I molecules could serve not only to block recognition by natural killer cells, but also to guide virus-specific HLA-C- and possibly HLA-G-restricted cytotoxic T-lymphocytes to their targets.
Figures


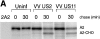
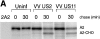
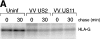
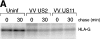
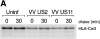
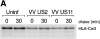
Similar articles
-
Human cytomegalovirus-encoded US2 differentially affects surface expression of MHC class I locus products and targets membrane-bound, but not soluble HLA-G1 for degradation.J Immunol. 2003 Dec 15;171(12):6757-65. doi: 10.4049/jimmunol.171.12.6757. J Immunol. 2003. PMID: 14662880
-
Subtle sequence variation among MHC class I locus products greatly influences sensitivity to HCMV US2- and US11-mediated degradation.Int Immunol. 2006 Jan;18(1):173-82. doi: 10.1093/intimm/dxh362. Epub 2005 Dec 16. Int Immunol. 2006. PMID: 16361314
-
Human cytomegalovirus gene products US3 and US6 down-regulate trophoblast class I MHC molecules.J Immunol. 2000 Jan 15;164(2):805-11. doi: 10.4049/jimmunol.164.2.805. J Immunol. 2000. PMID: 10623826
-
Identifying the ERAD ubiquitin E3 ligases for viral and cellular targeting of MHC class I.Mol Immunol. 2015 Dec;68(2 Pt A):106-11. doi: 10.1016/j.molimm.2015.07.005. Epub 2015 Jul 22. Mol Immunol. 2015. PMID: 26210183 Free PMC article. Review.
-
The HCMV gene products US2 and US11 target MHC class I molecules for degradation in the cytosol.Curr Top Microbiol Immunol. 2002;269:37-55. doi: 10.1007/978-3-642-59421-2_3. Curr Top Microbiol Immunol. 2002. PMID: 12224515 Review.
Cited by
-
A Coevolutionary Arms Race between Hosts and Viruses Drives Polymorphism and Polygenicity of NK Cell Receptors.Mol Biol Evol. 2015 Aug;32(8):2149-60. doi: 10.1093/molbev/msv096. Epub 2015 Apr 23. Mol Biol Evol. 2015. PMID: 25911231 Free PMC article.
-
The New Kid on the Block: HLA-C, a Key Regulator of Natural Killer Cells in Viral Immunity.Cells. 2021 Nov 10;10(11):3108. doi: 10.3390/cells10113108. Cells. 2021. PMID: 34831331 Free PMC article. Review.
-
Reporter-Based Screens for the Ubiquitin/Proteasome System.Front Chem. 2020 Feb 11;8:64. doi: 10.3389/fchem.2020.00064. eCollection 2020. Front Chem. 2020. PMID: 32117887 Free PMC article. Review.
-
Human cytomegalovirus US2 endoplasmic reticulum-lumenal domain dictates association with major histocompatibility complex class I in a locus-specific manner.J Virol. 2001 Jun;75(11):5197-204. doi: 10.1128/JVI.75.11.5197-5204.2001. J Virol. 2001. PMID: 11333901 Free PMC article.
-
Free major histocompatibility complex class I heavy chain is preferentially targeted for degradation by human T-cell leukemia/lymphotropic virus type 1 p12(I) protein.J Virol. 2001 Jul;75(13):6086-94. doi: 10.1128/JVI.75.13.6086-6094.2001. J Virol. 2001. PMID: 11390610 Free PMC article.
References
-
- Hammer A, Hutter H, Dohr G. HLA class I expression on the materno-fetal interface. Am J Reprod Immunol. 1997;38:150–157. - PubMed
-
- Hutter H, Hammer A, Blaschitz A, Hartmann M, Ebbesen P, Dohr G, Ziegler A, Uchanska-Ziegler B. Expression of HLA class I molecules in human first trimester and term placenta trophoblast. Cell Tissue Res. 1996;286:439–447. - PubMed
-
- King A, Boocock C, Sharkey AM, Gardner L, Beretta A, Siccardi AG, Loke YW. Evidence for the expression of HLA-C class I mRNA and protein by human first trimester trophoblast. J Immunol. 1996;156:2068–2076. - PubMed
-
- Sernee, M., H. Ploegh, and D. Schust. 1998. Why HLA-G and HLA-A cross-react: Epitope mapping of two common anti-MHC class I antibodies. Mol. Immunol. In press. - PubMed
-
- Yelavarthi K, Fishback J, Hunt J. Analysis of HLA-G mRNA in human placental and extraplacental membrane cells by in situ hybridization. J Immunol. 1991;146:2847–2854. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

