Transendothelial migration of megakaryocytes in response to stromal cell-derived factor 1 (SDF-1) enhances platelet formation
- PMID: 9687531
- PMCID: PMC2212480
- DOI: 10.1084/jem.188.3.539
Transendothelial migration of megakaryocytes in response to stromal cell-derived factor 1 (SDF-1) enhances platelet formation
Abstract
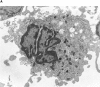
Although thrombopoietin has been shown to promote megakaryocyte (MK) proliferation and maturation, the exact mechanism and site of platelet formation are not well defined. Studies have shown that MKs may transmigrate through bone marrow endothelial cells (BMEC), and release platelets within the sinusoidal space or lung capillaries. In search for chemotactic factor(s) that may mediate transmigration of MKs, we have discovered that mature polyploid MKs express the G protein-coupled chemokine receptor CXCR4 (Fusin, LESTR). Therefore, we explored the possibility that stromal cell-derived factor 1 (SDF-1), the ligand for CXCR4, may also induce transendothelial migration of mature MKs. SDF-1, but not other CXC or CC chemokines, was able to mediate MK migration (ED50 = 125 pmol/liter). The MK chemotaxis induced by SDF-1 was inhibited by the CXCR4-specific mAb (12G5) and by pertussis toxin, demonstrating that signaling via the G protein-coupled receptor CXCR4 was necessary for migration. SDF-1 also induced MKs to migrate through confluent monolayers of BMEC by increasing the affinity of MKs for BMEC. Activation of BMEC with interleukin 1beta resulted in a threefold increase in the migration of MKs in response to SDF-1. Neutralizing mAb to the endothelial-specific adhesion molecule E-selectin blocked the migration of MKs by 50%, suggesting that cellular interaction of MKs with BMEC is critical for the migration of MKs. Light microscopy and ploidy determination of transmigrated MKs demonstrated predominance of polyploid MKs. Virtually all platelets generated in the lower chamber also expressed CXCR4. Platelets formed in the lower chamber were functional and expressed P-selectin (CD62P) in response to thrombin stimulation. Electron microscopy of the cells that transmigrated through the BMEC monolayers in response to SDF-1 demonstrated the presence of intact polyploid MKs as well as MKs in the process of platelet formation. These results suggest that SDF-1 is a potent chemotactic factor for mature MKs. Expression of CXCR4 may be the critical cellular signal for transmigration of MKs and platelet formation.
Figures
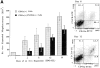






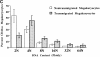
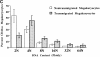
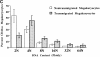


References
-
- Kaushansky K, Lok S, Holly RD, Broudy VC, Lin N, Bailey MC, Forstrom JW, Buddle MM, Oort PJ, Hagen FS, et al. Promotion of megakaryocyte progenitor expansion and differentiation by the c-Mpl ligand thrombopoietin. Nature. 1994;369:568–571. - PubMed
-
- Wendling F, Maraskovsky E, Debili N, Florindo C, Teepe M, Titeux M, Methia N, Breton-Gorius J, Cosman D, Vainchenker W. cMpl ligand is a humoral regulator of megakaryocytopoiesis. Nature. 1994;369:571–574. - PubMed
-
- Zucker-Franklin D, Kaushansky K. Effect of thrombopoietin on the development of megakaryocytes and platelets: an ultrastructural analysis. Blood. 1996;88:1632–1638. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

