The Vav-Rac1 pathway in cytotoxic lymphocytes regulates the generation of cell-mediated killing
- PMID: 9687532
- PMCID: PMC2212464
- DOI: 10.1084/jem.188.3.549
The Vav-Rac1 pathway in cytotoxic lymphocytes regulates the generation of cell-mediated killing
Abstract
The Rac1 guanine nucleotide exchange factor, Vav, is activated in hematopoietic cells in response to a large variety of stimuli. The downstream signaling events derived from Vav have been primarily characterized as leading to transcription or transformation. However, we report here that Vav and Rac1 in natural killer (NK) cells regulate the development of cell-mediated killing. There is a rapid increase in Vav tyrosine phosphorylation during the development of antibody-dependent cellular cytotoxicity and natural killing. In addition, overexpression of Vav, but not of a mutant lacking exchange factor activity, enhances both forms of killing by NK cells. Furthermore, dominant-negative Rac1 inhibits the development of NK cell-mediated cytotoxicity by two mechanisms: (a) conjugate formation between NK cells and target cells is decreased; and (b) those NK cells that do form conjugates have decreased ability to polarize their granules toward the target cell. Therefore, our results suggest that in addition to participating in the regulation of transcription, Vav and Rac1 are pivotal regulators of adhesion, granule exocytosis, and cellular cytotoxicity.
Figures



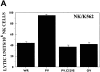


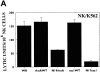


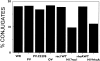


References
-
- Bustelo XR. The VAV family of signal transduction molecules. Crit Rev Oncog. 1996;7:65–88. - PubMed
-
- Collins T, Deckert M, Altman A. Views on Vav. Immunol Today. 1997;18:221–225. - PubMed
-
- Bustelo XR, Ledbetter JA, Barbacid M. Product of the vavproto-oncogene defines a new class of tyrosine protein kinase substrates. Nature. 1992;356:68–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

