Regulatory volume decrease and intracellular Ca2+ in murine neuroblastoma cells studied with fluorescent probes
- PMID: 9689024
- PMCID: PMC2525742
- DOI: 10.1085/jgp.112.2.145
Regulatory volume decrease and intracellular Ca2+ in murine neuroblastoma cells studied with fluorescent probes
Abstract
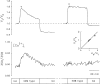
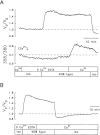
The possible role of Ca2+ as a second messenger mediating regulatory volume decrease (RVD) in osmotically swollen cells was investigated in murine neural cell lines (N1E-115 and NG108-15) by means of novel microspectrofluorimetric techniques that allow simultaneous measurement of changes in cell water volume and [Ca2+]i in single cells loaded with fura-2. [Ca2+]i was measured ratiometrically, whereas the volume change was determined at the intracellular isosbestic wavelength (358 nm). Independent volume measurements were done using calcein, a fluorescent probe insensitive to intracellular ions. When challenged with approximately 40% hyposmotic solutions, the cells expanded osmometrically and then underwent RVD. Concomitant with the volume response, there was a transient increase in [Ca2+]i, whose onset preceded RVD. For hyposmotic solutions (up to approximately -40%), [Ca2+]i increased steeply with the reciprocal of the external osmotic pressure and with the cell volume. Chelation of external and internal Ca2+, with EGTA and 1,2-bis-(o -aminophenoxy) ethane-N,N,N ',N '-tetraacetic acid (BAPTA), respectively, attenuated but did not prevent RVD. This Ca2+-independent RVD proceeded even when there was a concomitant decrease in [Ca2+]i below resting levels. Similar results were obtained in cells loaded with calcein. For cells not treated with BAPTA, restoration of external Ca2+ during the relaxation of RVD elicited by Ca2+-free hyposmotic solutions produced an increase in [Ca2+]i without affecting the rate or extent of the responses. RVD and the increase in [Ca2+]i were blocked or attenuated upon the second of two approximately 40% hyposmotic challenges applied at an interval of 30-60 min. The inactivation persisted in Ca2+-free solutions. Hence, our simultaneous measurements of intracellular Ca2+ and volume in single neuroblastoma cells directly demonstrate that an increase in intracellular Ca2+ is not necessary for triggering RVD or its inactivation. The attenuation of RVD after Ca2+ chelation could occur through secondary effects or could indicate that Ca2+ is required for optimal RVD responses.
Figures









References
-
- Adorante JS, Cala PM. Mechanisms of regulatory volume decrease in nonpigmented human ciliary epithelial cell. Am J Physiol. 1995;268:C721–C731. - PubMed
-
- Altamirano J, Brodwick MS, Alvarez-Leefmans FJ. Regulatory volume decrease is independent of internal calcium changes in N1E115 and NG108 cells. Biophys J. 1996;70:A413. . (Abstr.)
-
- Alvarez-Leefmans FJ, Altamirano J, Crowe WE. Use of ion-selective microelectrodes and fluorescent probes to measure cell volume. Methods Neurosci. 1995;27:361–391.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

