Effect of sarcoplasmic reticulum (SR) calcium content on SR calcium release elicited by small voltage-clamp depolarizations in frog cut skeletal muscle fibers equilibrated with 20 mM EGTA
- PMID: 9689025
- PMCID: PMC2525743
- DOI: 10.1085/jgp.112.2.161
Effect of sarcoplasmic reticulum (SR) calcium content on SR calcium release elicited by small voltage-clamp depolarizations in frog cut skeletal muscle fibers equilibrated with 20 mM EGTA
Erratum in
- J Gen Physiol 1998 Nov;112(5):649
Abstract
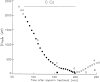
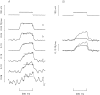
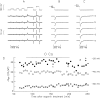
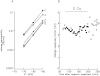
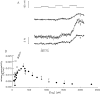
Cut muscle fibers from Rana temporaria (sarcomere length, 3.5-3.9 micro(m); 14-16 degreesC) were mounted in a double Vaseline-gap chamber and equilibrated with an external solution that contained tetraethyl ammonium- gluconate and an internal solution that contained Cs as the principal cation, 20 mM EGTA, and 0 Ca. Fibers were stimulated with a voltage-clamp pulse protocol that consisted of pulses to -70, -65, -60, -45, and -20 mV, each separated by 400-ms periods at -90 mV. The change in total Ca that entered into the myoplasm (Delta[CaT]) and the Ca content of the SR ([CaSR]) were estimated with the EGTA/phenol red method (Pape, P.C., D.-S. Jong, and W.K. Chandler. 1995. J. Gen. Physiol. 106:259-336). Fibers were stimulated with the pulse protocol, usually every 5 min, so that the resting value of [CaSR] decreased from its initial value of 1,700-2, 300 microM to values near or below 100 microM after 18-30 stimulations. Three main findings for the voltage pulses to -70, -65, and -60 mV are: (a) the depletion-corrected rate of Ca release (release permeability) showed little change when [CaSR] decreased from its highest level (>1,700 microM) to approximately 1,000 microM; (b) as [CaSR] decreased below 1,000 microM, the release permeability increased to a maximum level when [CaSR] was near 300 microM that was on average about sevenfold larger than the values observed for [CaSR] > 1,000 microM; and (c) as [CaSR] decreased from approximately 300 microM to <100 microM, the release permeability decreased, reaching half its maximum value when [CaSR] was approximately 110 microM on average. It was concluded that finding b was likely due to a decrease in Ca inactivation, while finding c was likely due to a decrease in Ca-induced Ca release.
Figures



References
-
- Armstrong CM, Bezanilla F, Horowicz P. Twitches in the presence of ethylene glycol-bis(β-aminoethylether)-N,N′-tetraacetic acid. Biochim Biophys Acta. 1972;267:605–608. - PubMed
-
- Baylor SM, Chandler WK, Marshall MW. Arzenazo III signals in singly dissected frog twitch fibers. J Physiol (Camb) 1979;287:23–24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

