Desensitization of mouse nicotinic acetylcholine receptor channels. A two-gate mechanism
- PMID: 9689026
- PMCID: PMC2525745
- DOI: 10.1085/jgp.112.2.181
Desensitization of mouse nicotinic acetylcholine receptor channels. A two-gate mechanism
Abstract
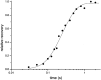
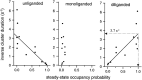
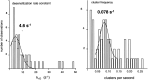
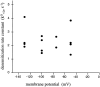
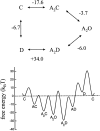
The rate constants of acetylcholine receptor channels (AChR) desensitization and recovery were estimated from the durations and frequencies of clusters of single-channel currents. Diliganded-open AChR desensitize much faster than either unliganded- or diliganded-closed AChR, which indicates that the desensitization rate constant depends on the status of the activation gate rather than the occupancy of the transmitter binding sites. The desensitization rate constant does not change with the nature of the agonist, the membrane potential, the species of permeant cation, channel block by ACh, the subunit composition (epsilon or gamma), or several mutations that are near the transmitter binding sites. The results are discussed in terms of cyclic models of AChR activation, desensitization, and recovery. In particular, a mechanism by which activation and desensitization are mediated by two distinct, but interrelated, gates in the ion permeation pathway is proposed.
Figures
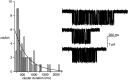




References
-
- Akabas MH, Kaufmann C, Archdeacon P, Karlin A. Identification of acetylcholine receptor channel-lining residues in the entire M2 segment of the α subunit. Neuron. 1994;13:919–927. - PubMed

