Muscarinic K+ channel in the heart. Modal regulation by G protein beta gamma subunits
- PMID: 9689027
- PMCID: PMC2525744
- DOI: 10.1085/jgp.112.2.199
Muscarinic K+ channel in the heart. Modal regulation by G protein beta gamma subunits
Abstract
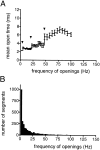
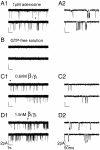
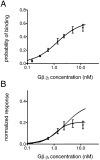
The membrane-delimited activation of muscarinic K+ channels by G protein beta gamma subunits plays a prominent role in the inhibitory synaptic transmission in the heart. These channels are thought to be heterotetramers comprised of two homologous subunits, GIRK1 and CIR, both members of the family of inwardly rectifying K+ channels. Here, we demonstrate that muscarinic K+ channels in neonatal rat atrial myocytes exhibit four distinct gating modes. In intact myocytes, after muscarinic receptor activation, the different gating modes were distinguished by differences in both the frequency of channel opening and the mean open time of the channel, which accounted for a 76-fold increase in channel open probability from mode 1 to mode 4. Because of the tetrameric architecture of the channel, the hypothesis that each of the four gating modes reflects binding of a different number of Gbeta gamma subunits to the channel was tested, using recombinant Gbeta1 gamma5. Gbeta1 gamma5 was able to control the equilibrium between the four gating modes of the channel in a manner consistent with binding of Gbeta gamma to four equivalent and independent sites in the protein complex. Surprisingly, however, Gbeta1 gamma5 lacked the ability to stabilize the long open state of the channel that is responsible for the augmentation of the mean open time in modes 3 and 4 after muscarinic receptor stimulation. The modal regulation of muscarinic K+ channel gating by Gbeta gamma provides the atrial cells with at least two major advantages: the ability to filter out small inputs from multiple membrane receptors and yet the ability to create the gradients of information necessary to control the heart rate with great precision.
Figures




Similar articles
-
Cytoplasmic unsaturated free fatty acids inhibit ATP-dependent gating of the G protein-gated K(+) channel.J Gen Physiol. 2000 Mar;115(3):287-304. doi: 10.1085/jgp.115.3.287. J Gen Physiol. 2000. PMID: 10694258 Free PMC article.
-
Na+ activation of the muscarinic K+ channel by a G-protein-independent mechanism.J Gen Physiol. 1996 Nov;108(5):381-91. doi: 10.1085/jgp.108.5.381. J Gen Physiol. 1996. PMID: 8923264 Free PMC article.
-
Synergistic activation of G protein-gated inwardly rectifying potassium channels by the betagamma subunits of G proteins and Na(+) and Mg(2+) ions.J Gen Physiol. 1999 Nov;114(5):673-84. doi: 10.1085/jgp.114.5.673. J Gen Physiol. 1999. PMID: 10532964 Free PMC article.
-
G-protein mediated gating of inward-rectifier K+ channels.Eur J Biochem. 2000 Oct;267(19):5830-6. doi: 10.1046/j.1432-1327.2000.01670.x. Eur J Biochem. 2000. PMID: 10998041 Review.
-
Roles of G proteins in coupling of receptors to ionic channels and other effector systems.Crit Rev Biochem Mol Biol. 1990;25(4):225-44. doi: 10.3109/10409239009090610. Crit Rev Biochem Mol Biol. 1990. PMID: 2171876 Review.
Cited by
-
Single channel studies of inward rectifier potassium channel regulation by muscarinic acetylcholine receptors.J Gen Physiol. 2000 Nov;116(5):645-52. doi: 10.1085/jgp.116.5.645. J Gen Physiol. 2000. PMID: 11055993 Free PMC article.
-
NMR analyses of the Gbetagamma binding and conformational rearrangements of the cytoplasmic pore of G protein-activated inwardly rectifying potassium channel 1 (GIRK1).J Biol Chem. 2011 Jan 21;286(3):2215-23. doi: 10.1074/jbc.M110.160754. Epub 2010 Nov 12. J Biol Chem. 2011. PMID: 21075842 Free PMC article.
-
Functional characterization of a small conductance GIRK channel in rat atrial cells.Biophys J. 2004 Nov;87(5):3122-36. doi: 10.1529/biophysj.103.039487. Biophys J. 2004. PMID: 15507689 Free PMC article.
-
Emergence of ion channel modal gating from independent subunit kinetics.Proc Natl Acad Sci U S A. 2016 Sep 6;113(36):E5288-97. doi: 10.1073/pnas.1604090113. Epub 2016 Aug 22. Proc Natl Acad Sci U S A. 2016. PMID: 27551100 Free PMC article.
-
Kinetic modeling of Na(+)-induced, Gbetagamma-dependent activation of G protein-gated K(+) channels.J Mol Neurosci. 2005;25(1):7-19. doi: 10.1385/JMN:25:1:007. J Mol Neurosci. 2005. PMID: 15781962
References
-
- Breitwieser GE, Szabo G. Uncoupling of cardiac muscarinic and β-adrenergic receptors from ion channels by a guanine nucleotide analogue. Nature. 1985;317:538–540. - PubMed
-
- Clapham DE, Neer EJ. New roles for G-protein βγ-dimers in transmembrane signalling. Nature. 1993;365:403–406. - PubMed
-
- Colquhoun D, Hawkes AG. On the stochastic properties of single ion channels. Proc R Soc Ser B. 1981;211:205–235. - PubMed
-
- Colquhoun, D., and A.G. Hawkes. 1983. The principles of the stochastic interpretation of ion-channel mechanisms. In Single Channel Recording. B. Sakmann and E. Neher, editors. Plenum Publishing Corp., New York. 135–175.

