beta-Lactam synthetase: a new biosynthetic enzyme
- PMID: 9689037
- PMCID: PMC21295
- DOI: 10.1073/pnas.95.16.9082
beta-Lactam synthetase: a new biosynthetic enzyme
Abstract
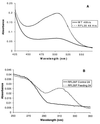
The principal cause of bacterial resistance to penicillin and other beta-lactam antibiotics is the acquisition of plasmid-encoded beta-lactamases, enzymes that catalyze hydrolysis of the beta-lactam bond and render these antibiotics inactive. Clavulanic acid is a potent inhibitor of beta-lactamases and has proven clinically effective in combating resistant infections. Although clavulanic acid and penicillin share marked structural similarities, the biosyntheses of their bicyclic nuclei are wholly dissimilar. In contrast to the efficient iron-mediated oxidative cyclization of a tripeptide to isopenicillin N, the critical beta-lactam ring of clavulanic acid is demonstrated to form by intramolecular closure catalyzed by a new type of ATP/Mg2+-dependent enzyme, a beta-lactam synthetase (beta-LS). Insertional inactivation of its encoding gene in wild-type Streptomyces clavuligerus resulted in complete loss of clavulanic acid production and the accumulation of N2-(carboxyethyl)-L-arginine (CEA). Chemical complementation of this blocked mutant with authentic deoxyguanidinoproclavaminic acid (DGPC), the expected product of the beta-LS, restored clavulanic acid synthesis. Finally, overexpression of this gene gave the beta-LS, which was shown to mediate the conversion of CEA to DGPC in the presence of ATP/Mg2+. Primary amino acid sequence comparisons suggest that this mode of beta-lactam formation could be more widely spread in nature and mechanistically related to asparagine synthesis.
Figures
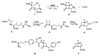

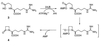
References
-
- Service R F. Science. 1995;270:724–727. - PubMed
-
- Neu H C. Science. 1992;257:1064–1073. - PubMed
-
- Baggaley K H, Brown A G, Schofield C J. Nat Prod Rep. 1997;14:309–333. - PubMed
-
- Marsden A F A, Wilkinson B, Cortes J, Dunster N J, Staunton J, Leadlay P F. Science. 1998;279:199–202. - PubMed
-
- Jacobsen J R, Hutchinson C R, Cane D E, Khosla C. Science. 1997;277:367–369. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

